Source: eRA Commons Website
Seek NIH Prior Approval (If Needed)
Information on how to electronically obtain prior approval for certain activities from the awarding agency, using the Prior Approval features in eRA Commons.
Prior Approval is the process wherein an applicant or grantee must have written approval by an authorized official to undertake a certain activity. Also see, the FDP Prior Approval Matrix.
eRA now supports three kinds of Prior Approval requests post-award:
Basic Tasks (step-by-step instructions from the online help)*
- Requesting a No Cost Extension via Prior Approval
- Carryover Request Process
- Requesting a Change of PD/PI on a Grant
* You must be logged into eRA Commons with appropriate role(s) to complete these activities.
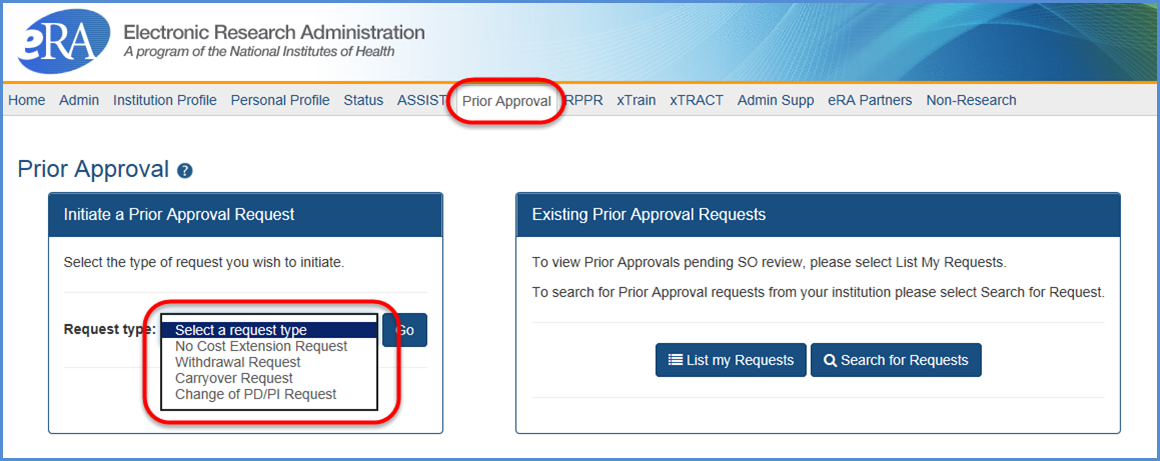
Main Screenshot
Click on thumbnail image to expand to full view.
Figure 1: The Prior Approval screen showing the options for request types
Policy
- NIH Grants Policy Statement: Prior Approval Requirements
Summary of Actions Requiring NIH Prior Approval
Source: Grants Policy Statement (8.1.2) (for budget periods beginning on or after October 1, 2021)
| NIH prior approval is required for | Under the following circumstances |
|---|---|
| Additional no-cost extension, extension greater than 12 months, or late notification of initial no-cost extension (8.1.2.1) | All instances. |
| A&R (8.1.2.2) |
Rebudgeting into A&R costs that would exceed 25 percent of the total approved budget for a budget period. If rebudgeting would not meet this threshold but would result in a change in scope. Addition of a Major single A&R project. |
| Capital expenditures (construction, land, or building acquisition) (8.1.2.3) | All instances. Also, any proposals to convey, transfer, assign, mortgage, lease, or in any other manner encumber real property acquired with NIH grant funds. |
| Carryover of unobligated balances (8.1.2.4) | If the NoA indicates that the recipient does not have the authority to automatically carry over unobligated balances. |
| Change in scope (8.1.2.5) | All instances. |
| Change in status of the PD/PI or senior/key personnel named in the NoA (8.1.2.6) | A significant change in the status including but not limited to withdrawal from the project; absence for any continuous period of 3 months or more; reduction of the level of effort devoted to project by 25 percent or more from what was approved in the initial competing year award. |
| Change of recipient organization (8.1.2.7) | All instances. |
| Change of recipient organization status (8.1.2.8) | All instances. |
| Deviation from award terms and conditions (8.1.2.9) | All instances. Includes undertaking any activities disapproved or restricted as a condition of the award. |
| Foreign component added to a grant to a domestic or foreign organization (8.1.2.10) | All instances. |
| Make subawards based on fixed amounts (8.1.2.11) | All instances |
| Need for additional NIH funding (8.1.2.12 and 8.1.2.13) | All instances, including extension of a final budget period of a project period with additional funds. |
| Pre-award costs (8.1.2.14) | More than 90 days before effective date of the initial budget period of a new or competing continuation award; always at the recipient's own risk. |
| Rebudgeting funds from trainee costs (8.1.2.15) | All instances. |
| Rebudgeting of funds between construction and non-construction work (8.1.2.16) | All instances. |
| Retention of research grant funds when CDA awarded (8.1.2.17) | All instances. |