NIH Update
February 2, 2020
Message from NIH
FY 2021 Fiscal Policies for Grant Awards
NIH issued guidance for NIH Fiscal Operations for FY 2021, including the following policies:
- FY 2021 Funding Levels: Non-competing continuation awards made in FY 2021 will generally be issued at the commitment level indicated on the Notice of Award.
- Ruth L. Kirschstein National Research Service Awards (NRSA): NIH will increase NRSA stipends by approximately two percent for predocs and two percent for postdocs. More information provided in post below.
- Salary Limits: New salary cap is set at $199,300. More information provided in post below.
- Next Generation Researchers Initiative Policy: NIH will prioritize meritorious R01-equivalent applications from Early Stage Investor (ESI) PD/PIs.
- Other Legislative Mandates: Other statutory requirements that limits or conditions the use of funds on NIH grant, cooperative agreement, and contract awards for FY 2021.
For additional guidance and details, see NOT-OD-21-058.
______________________________________________________________________________________
NIH Update
January 28, 2020
Message from NIH
Writing An Effective “K” Application: A Video Guide
Do you need some guidance on preparing a K Award application for the NIH? Dr. Kay Lund, Director of Division of Biomedical Research Workforce, gives some great tips in a 25-minute YouTube video, “Writing an Effective ‘K’ Application.” It is designed for junior investigators and those who assist in the preparation of the scientific portions of an application.
The video covers points including:
- Currently active K award funding opportunity announcements & where to find them
- A breakdown of the different K awards
- Planning tips
- Application requirements
- Review criteria
You will also learn how to avoid the most common mistakes in writing K applications, as well as some typical misconceptions about the review process.
_______________________________________________________________________
NIH Update
January 22, 2020
Message from NIH
Enhancing Diversity at NIH-Funded Conferences
A recent Harvard Business Review article noted that the gap between awareness and action when it comes to gender equity is ‘gender fatigue’ – a “phenomenon of simultaneously acknowledging that gender inequality exists in general while denying that it exists in one’s immediate work environment.” And the article questions why organizations are not making more progress towards gender equity, while making recommendations to avoid the mismatch.
At NIH, we have and continue to focus not just on gender equity but on ensuring greater diversity in all aspects of the biomedical workforce. This means, that along with women, members of racial and/or ethnic minority groups, people with disabilities, and those from disadvantaged backgrounds are also included. To help ensure that the nation remains a global leader in scientific discovery and innovation, NIH needs the richness and breadth of varied perspectives that comes from having a pool of highly talented scientists from diverse backgrounds.
Read the full article at NIH's Open Mike blog.
__________________________________________________________________
NIH Update
January 6, 2020
Message from NIH
Explore RePORTER’s State Map Visualizations
Ever find yourself wondering what and how much research NIH supports near you? Check out what the modernized RePORTER site has to offer in three easy steps! RePORTER’s main search page offers a new map visualization, highlighting active NIH projects by state.
_______________________________________________________________________
NIH Update
January 5, 2020
Message from NIH
All About Grants Podcast: Human Subjects’ Research Post-Award
So you have confirmed that you are doing human subjects’ research after listening to the first podcast in our human subject mini-series. And you have a clear human subjects’ protection and monitoring plan developed for your application after tuning in to the second episode in the series. Now, what should you keep in mind after the award is made?
The latest NIH All About Grants podcast episode delves into just this issue (MP3 / Transcript). Lyndi Lahl, R.N., an NIH Human Subjects’ Officer, joins us (and her dog too!) in this final episode of this human subjects’ research mini-series. Tune in for tips about important post-award requirements, what’s needed for annual progress reporting, engaging your IRB and NIH when a protocol change is needed, the difference between adverse events and unanticipated problems, and much more.
________________________________________________________________________
NIH Update
December 2, 2020
Message from NIH
Federal Financial Report (FFR) Required to be Submitted in the Payment Management System (PMS)
Recipients will be required to submit the SF-425 Federal Financial Report, a statement of expenditures associated with their award, to the Payment Management System (PMS) instead of eRA Commons, effective January 1, 2021 (see NIH Guide Notice NOT-OD-20-127).
The change in submission requirement is part of an HHS initiative to consolidate FFR reporting from all the HHS Operating Divisions into PMS.
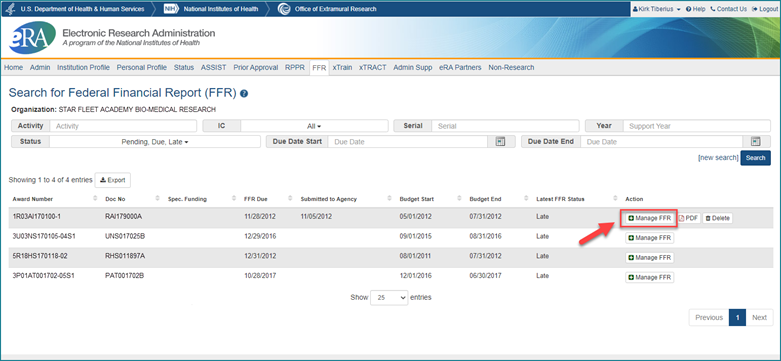
To make it seamless for recipients, clicking on the new Manage FFR button next to a particular grant on the Search for Federal Financial Report (FFR) screen in eRA Commons will take the recipient directly to PMS, where the recipient will log in and be taken straight to the Federal Financial Report - Details screen for that grant in PMS. While the interface will appear new to the recipients, the questions remain the same. Once the form is completed in PMS, the recipient will submit it to the agency. Recipients will still be able to see the status of their submission in the eRA Commons/FFR module.
Figure 1: The Manage FFR button in eRA Commons/FFR that will take the user to the Payment Management System
Note that recipients should register with PMS and obtain log in credentials prior to submitting an FFR. Recipient organizations should be familiar with PMS as they use the tool to draw down grant funds. Also note that recipients who have FFRs in the eRA Commons/FFR module that are a work-in-progress as of Jan. 1, 2021, will need to start over in PMS.
The change to FFR submission requirements does not affect the timeline. Therefore, FFR due dates, as outlined in the NIH Grants Policy Statement, 8.4.1.5.2 and 8.6.1, remain unchanged. FFRs that are submitted prior to January 1, 2021 will use the FFR module in eRA Commons.
A second guide notice will be issued soon. Training sessions offered by PMS for recipients will be posted at this link as they become available: https://pms.psc.gov/training/gr-ffr-training.html
__________________________________________________________________________________________________________
NIH Update
December 1, 2020
Message from NIH
New Human Research Protection Training
Investigators and all key personnel involved in human subjects research are required to receive education in the protection of human subjects (see NOT-OD-00-039). One way to satisfy this requirement is by completing the newly launched Human Research Protection Training offered by the HHS Office for Human Research Protections (OHRP).
The training targets the broad research community including IRBs, investigators and key personnel, and anyone interested in the Common Rule. Access is free and viewers can print a completion certificate upon completing each of the 4 lessons.
__________________________________________________________________________________________________________
NSF Update
November 24, 2020
Message from NSF
New Proposal Types in Research.gov
Effective November 23, 2020, the National Science Foundation (NSF) enabled three new proposal types in the Research.gov Proposal Submission System and in the recently launched Research.gov demo site. These are the Rapid Response Research (RAPID), EArly-concept Grants for Exploratory Research (EAGER), and Research Advanced by Interdisciplinary Science and Engineering (RAISE) proposal types. New automated compliance checks and associated error and warning messages were also implemented.
In addition, based on feedback from the research community, NSF has removed the font type and font size automated compliance checks and compliance warning messages for Research.gov proposals to align with FastLane and NSF policy.
New and updated system-related Frequently Asked Questions (FAQs) are available on the Research.gov About Proposal Preparation and Submission page via the left navigation menu.
RAPID, EAGER, and RAISE Proposals
- Proposers can now select a RAPID, EAGER, or RAISE proposal in the Research.gov proposal creation wizard, in addition to the existing Research proposal option. These proposal types are also available in the Research.gov proposal preparation demo site.
- New automated compliance checks for RAPID, EAGER, and RAISE proposals have been added to Research.gov and are listed on the updated Research.gov Compliance Checklist dated November 23, 2020 on the Automated Compliance Checking of NSF Proposals page. Error messages prohibit proposal submission to NSF, whereas warning messages still permit proposal submission.
- Refer to the Proposal & Award Policies & Procedures Guide (PAPPG) (NSF 20-1) for RAPID, EAGER, and RAISE proposal requirements.
Removal of Font Type and Font Size Compliance Checks
- Although the automated compliance checks and associated compliance warnings for font type and font size have been removed, proposals may still be returned without review if the font type or font size is not compliant with the PAPPG Chapter II.B.2.a.
- Refer to the updated Research.gov Compliance Checklist dated November 23, 2020 on the Automated Compliance Checking of NSF Proposals page for a complete listing of the current automated proposal compliance checks for Research.gov proposals.
What's Ahead?
Research.gov is being developed incrementally, and features are expanding to support the transition of all proposal preparation and submission functionality from FastLane to Research.gov in accordance with NSF Important Notice 147: Research.gov Implementation Update issued September 22, 2020. Please refer to the new Proposal Submission Capabilities list on the Research.gov About Proposal Preparation and Submission page left navigation menu to see what is in development.
Questions?
If you have IT system-related questions, please contact the NSF Help Desk at 1-800-381-1532 (7:00 AM - 9:00 PM ET; Monday - Friday except federal holidays) or via rgov@nsf.gov. Policy-related questions should be directed to policy@nsf.gov.
We look forward to receiving your Research.gov RAPID, EAGER, and RAISE proposals!
Regards,
Research.gov Team at the National Science Foundation
__________________________________________________________________________________________________________
NIH Update
November 19, 2020
Message from NIH
Should We Keep Meeting This Way?
How will study sections meet in the future? NIH peer review depends on robust meetings where groups of scientists, through vigorous discussion, identify the applications of highest merit. For the last 75 years, until last March, nearly all chartered review committee meetings were held in-person. Today, in response to the pandemic, 90% of all CSR review meetings are run as video (“Zoom”) meetings. CSR is taking steps now so that when all options are back on the table, we can make informed choices about how best to convene review meetings.
For detailed information, visit NIH’s Extramural News. To read the details of the NIH’s analyses, thus far, of the survey responses received from 3,000 NIH reviewers and support staff, view the CSR Analysis of Zoom in Reviews.
__________________________________________________________________________________________________________
NIH Update
November 18, 2020
Message from NIH
“All About Grants” Podcast – Alternatives to Animals
Your experimental designs are coming into focus. Sample sizes…power analyses…and treatment conditions, oh my! And, all throughout, perhaps laboratory animals are needed. But, are they? Can you actually replace them and still rigorously test the hypothesis? If not, maybe the protocol can be refined in such a way to reduce their overall numbers, while still ensuring their humane care and use?
Considering alternatives to animals in your application is the topic of our next NIH All About Grants podcast. Drs. Neera Gopee with the NIH Office of Laboratory Animal Welfare and Christine Livingston with the National Center for Advancing Translational Sciences join us for this conversation (MP3 / Transcript). We will go into the 3Rs (replace, refine, and reduce), helpful resources for relevant policies, what’s needed for the vertebrate animal section, role for IACUCs and peer review, as well as organoids, in silico models, and other alternatives…oh my again!
On a related note, keep an eye out for recommendations coming from the Advisory Committee to the NIH Director working group on Enhancing Rigor, Transparency, and Translatability in Animal Research this December. Part of their charge is validating alternative models to animal research as well as considering benefits and burdens of registering animal studies. Their recommendations will also encompass public feedback in response to a Request for Information (NOT-OD-20-130) released this summer (see this NIH Open Mike blog post for more).
__________________________________________________________________________________________________________
Federal Terms & Conditions Update
November 12, 2020
Message from NSF
Revision of the Research Terms and Conditions
The Office of Management and Budget (OMB) mandated awarding agencies adopt recent revisions to 2 CFR 200: Uniform Guidance. Agency implementation statements provide specific details on how participating agencies are implementing the revised Research Terms and Conditions (RTCs). In accordance with this requirement, the updated RTCs, which implement the changes to 2 CFR 200, will be fully effective beginning November 12, 2020. This includes all supplemental appendices (Appendix A: Prior Approval Matrix, Appendix B: Subaward Requirements, and Appendix C: National Policy Requirements).
- Research Terms and Conditions (RTC) Agency Implementation Statements - November 12, 2020
- RTC Overlay to 2 CFR 200 - November 12, 2020
- RTC Appendix A Prior Approval Matrix - November 12, 2020
- RTC Appendix B Subaward Requirements - November 12, 2020
- RTC Appendix C National Policy Requirements - November 12, 2020
AGENCY SPECIFIC REQUIREMENTS
- DOC - 11/20
- DOE - 11/20
- HHS/NIH - 11/20
- NASA - 11/20
- NSF - 11/20 (Significant Changes in 11/20), 10/20 (Significant Changes in 10/20)
- USDA/NIFA - 11/20
RESEARCH TERMS AND CONDITIONS 2008-2019 (INCLUDING SUPPORTING DOCUMENTS)
Federal Register Notices
Agency Implementation Statements
RTC Overlay to 2 CFR 200
- March 2017 (side by side with 2 CFR 200)
- June 2011 (side by side with A-110)
- July 2008 (side by side with A-110)
RTC Appendix A - Prior Approval Matrix
RTC Appendix B - Subaward Requirements
RTC Appendix C - National Policy Requirements
AGENCY SPECIFIC REQUIREMENTS
- DHS - 12/18
- DOC - 11/17, 10/17, 2/13, 10/08
- DOD/AFOSR - 3/12, 7/08
- DOD/AMRAA - 10/11, 7/08
- DOD/ARO - 6/09, 7/08
- DOD/ONR - 2/11, 10/10, 7/08
- DOE - 4/17, 7/08
- EPA - 3/14, 9/13, 1/13, 10/12, 2/11, 11/10, 6/09, 7/08
- HHS/NIH - 4/17, 12/10, 7/08
- NASA - 9/19, 10/17, 7/08
- NSF - 2/19 (Significant Changes in 2/19), 10/18 (Significant Changes in 10/18), 5/18 (Significant Changes in 5/18), 3/18 (Significant Changes in 3/18), 10/17 (Technical Corrections to NSF ASR 10/17), 4/17, 3/14 (Significant Changes in 3/14), 2/14 (Significant Changes in 2/14), 1/13 (Significant Changes in 1/13), 02/12 (Significant Changes in 02/12), 10/10 (Significant Changes in 10/10), 1/10 (Significant Changes in 1/10), 1/09 (Significant Changes in 1/09), 7/08
- USDA/CSREES - 7/08
- USDA/NIFA - 6/17, 10/14, 11/13, 4/13, 2/13, 5/12, 4/12, 7/11, 10/10, 7/10, 11/09
__________________________________________________________________________________________________________
NSF Update
November 5, 2020
Message from NSF
Significant Changes to Revised NSF Terms and Conditions
Dear Colleagues:
I want to make you aware that the following sets of NSF Award Conditions have been updated for consistency with the revised 2 CFR §200, Uniform Administrative Requirements, Cost Principles, and Audit Requirements for Federal Awards.
- Grant General Conditions (GC-1);
- Cooperative Agreement Financial and Administrative Terms and Conditions (CAFATC); and
- Cooperative Agreement Modifications and Supplemental Financial and Administrative Terms and Conditions for Major Multi-User Research Facility Projects and Federally Funded Research and Development Centers.
The revised terms and conditions will apply to all new NSF awards and funding amendments to existing awards made on or after November 12, 2020.
The terms and conditions incorporate revised 2 CFR §200 coverage including: requirements for award termination and enforcement; compliance with Section 889 of the National Defense Authorization Act (NDAA) which prohibits the use of Federal assistance funding on certain telecommunications and video surveillance services or equipment; as well as other significant changes and clarifications. All sets of award conditions are accompanied by a summary of changes made to that document.
NSF will separately announce the release of the Agency Specific Requirements to the Research Terms and Conditions (RTC), as well as the Administration of NSF Conference or Group Travel Award Grant Conditions (FL-26).
Questions about NSF award conditions may be sent to policy@nsf.gov.
Jean Feldman
Head, Policy Office
Division of Institution & Award Support (DIAS)
Office of Budget, Finance and Award Management
National Science Foundation
__________________________________________________________________________________________________________
NIH Update
October 30, 2020
Message from NIH
How Can I Monitor Institutional Compliance With NIH’s Public Access Policy?
The NIH Public Access Compliance Monitor (PACM) provides an institution with the current compliance status of all journal articles that are associated with the institution that fall under the NIH Public Access Policy. This database is provided as a service to our awardees to help them track compliance, should they wish to use it. Check out the PACM User Guide to learn more!
__________________________________________________________________________________________________________
NIH Podcast
October 29, 2020
Message from NIH
“All About Grants” Podcast – Human Subjects’ Protection and Monitoring Plans
You’ve done your homework: read the requisite materials on human subjects, spoken with program staff at NIH, even listened to Part 1 of this podcast mini-series for some insights on how you know you are actually doing human subjects’ research. Now you’re ready to explain in your NIH grant application how research participants will be protected and monitored.
Dawn Corbett, NIH’s Inclusion Policy Officer, shares why human subjects’ protection and monitoring plans are important in this next NIH’s All About Grants podcast (MP3 / Transcript). We will discuss what should be included in these plans as part of your application, what should be left out, what are risks and what are benefits to study participants, how reviewers assess it all, and so much more.
__________________________________________________________________________________________________________
NSF Update
October 27, 2020
Message from NSF
Demo Site for Research.gov Proposal Preparation Now Available
Dear Colleagues:
We are pleased to announce that the National Science Foundation (NSF) has launched the Research.gov proposal preparation demonstration site. The new demo site offers proposers the opportunity to create proposals in Research.gov with the role of a Principal Investigator (PI) prior to preparing and submitting proposals in the actual Research.gov Proposal Submission System. We invite you to try the Research.gov proposal preparation features on the new demo site, such as:
-
Initiating Research proposals (other proposal types will be added to the demo site as they are enabled in the actual system):
- Single submissions from one organization
- Collaborative proposals with subawards
- Separately submitted collaborative proposals from multiple organizations
- Adding co-PIs, Senior Personnel, and Other Authorized Users (OAUs)
- Uploading required and optional proposal documents
- Creating budgets
- Checking proposal compliance
- Adding subawards
- Linking collaborative proposals
- Enabling Sponsored Project Officer (SPO)/Authorized Organizational Representative (AOR) access
What You Need to Know About the New Research.gov Demo Site
-
All users must sign in to Research.gov with an NSF ID or primary email address to access the demo site.
- Users without an NSF account (i.e., NSF ID) will first need to register for one to use the demo site.
- Users who already have an NSF ID must not register for another NSF ID for demo site use. As a reminder, each individual user of NSF systems (e.g., FastLane and Research.gov) should not have more than one NSF ID, per the NSF Proposal & Award Policies & Procedures Guide Chapter I.G.3.
- A red "Proposal Preparation Demo Site" banner is at the top of each demo site page to indicate the user is using the demo site.
- Each user will be given the PI role for demo site purposes only. No other user roles (e.g., SPO and AOR) are available on the demo site or are needed to use the demo site.
- The demo site does not support proposal submission to NSF and will not trigger any system-generated email notifications.
- Proposals created on the demo site will be deleted after six months. Neither NSF staff nor users will be able to access deleted proposal data from the demo site.
- Demo site proposals will not be available on the actual Research.gov Proposal Submission System, and proposals cannot be transferred between the demo site and the actual system.
- For further demo site details, please see the demo site Frequently Asked Questions (FAQs) available via the Research.gov About Proposal Preparation and Submission page left navigation menu. A set of topic-specific video tutorials is also available.
Accessing the Research.gov Proposal Preparation Demo Site
To access the Research.gov demo site, you must have an NSF account (i.e., NSF ID) and be signed in to Research.gov.
-
If you have an NSF account:
- Access Research.gov Demo Site: Prepare Proposals. (If you are not signed in, you will be prompted to sign in before accessing the demo site.)
-
If you do not have an NSF account:
- Open Research.gov
- Use the Register tab located on the top right of the screen to create an NSF account
- Input the requested account registration information
Important Note: Your primary registered email address will be used for NSF account notifications including password resets and can be used to sign in to Research.gov. Please ensure that you have ongoing access to your primary registered email (e.g., a personal email address), even if you change organizations. Refer to the Research.gov About Account Management page for additional registration guidance.
Retirement of FastLane Demo Site
The FastLane demo site has been retired, however, we encourage you to try the new Research.gov proposal preparation demo site. In accordance with Important Notice No. 147: Research.gov Implementation Update, NSF is taking proactive steps to incrementally move the preparation and submission of all proposals from FastLane to Research.gov.
Enhancements Coming Soon to Research.gov
Effective in late November 2020, NSF will:
-
Enable the following proposal types on Research.gov and on the new Research.gov proposal preparation demo site:
- Rapid Response Research (RAPID)
- EArly-concept Grants for Exploratory Research (EAGER)
- Research Advanced by Interdisciplinary Science and Engineering (RAISE)
- Remove the font type and font size compliance checks and associated warning messages per feedback from the research community.
Stay tuned for additional information about these updates in the next couple of weeks.
Questions? If you have IT system-related questions, please contact the NSF Help Desk at 1-800-381-1532 (7:00 AM - 9:00 PM ET; Monday - Friday except federal holidays) or via rgov@nsf.gov. Policy-related questions should be directed to policy@nsf.gov.
We look forward to seeing you on the new Research.gov proposal preparation demo site!
Regards,
National Science Foundation
__________________________________________________________________________________________________________
NIH Update
October 23, 2020
Message from NIH
Automated Trainee Diversity Report Required with RPPRs for Most T, K and Research Education Awards
An automatically generated Trainee Diversity Report will replace the manual report that signing officials are required to submit with Research Performance Progress Reports (RPPRs) for most institutional training, career development awards and research education grants, effective October 30, 2020. The automated report will leverage existing electronic demographic data entered by trainees in the Personal Profile of eRA Commons to minimize the need for manual data entry by recipients and reduce their burden.
The report can be generated by recipients from the xTrain and RPPR modules and the signing official (SO) will submit the RPPR with the automated report.
The eRA system will check whether the RPPRs for the specified grant types include an electronically generated Trainee Diversity Report. RPPRs lacking an electronically generated report will not be accepted.
Recipient organizations should encourage trainees to keep their information updated in their Personal Profiles, as the Trainee Diversity Report will be most accurate if the profile is complete.
Resources
- Guide Notice: NOT-OD-20-178
- Sample of the new report and more details: eRA news item
- Video tutorial: Electronic Trainee Diversity Report
___________________________________________________________________________________________________________
NIH Update
October 21, 2020
Message from NIH
NIH Will Continue to Accept Preliminary Data as Post-Submission Material Through August/October 2021 Councils
In recognition of the fact that COVID-19 may still be adversely affecting the ability of applicants to generate preliminary data, NIH will continue to accept a one-page update with preliminary data as post-submission materials for applications submitted for the August/October 2021 council (beginning with applications submitted for the January 25, 2021 due date for Summer 2021 review meetings), ONLY if the Funding Opportunity Announcement (FOA) used for submission allowed preliminary data in the application (NOT-OD-20-179).
The deadline for submitting all post-submission materials, including preliminary data, will be 30 days before the study section meeting. Because applications for emergency competitive revisions and urgent competitive revisions undergo expedited review, post-submission materials will not be accepted for those applications.
For a visualization of the peer review process and timelines during COVID-19, see the updated infographic.
See also How do I submit post-submission materials for my application?
___________________________________________________________________________________________________________
JustGrants Now Available
October 15, 2020
Message from Dept. of Justice
JustGrants Now Available
Starting today, October 15, 2020, the Justice Grants System (JustGrants) supports all functionalities necessary to support the essential stages of the grant management lifecycle. The Office of Community Oriented Policing Services (COPS Office), the Office of Justice Programs (OJP), and the Office on Violence Against Women (OVW)― transitioned to one grants management system (JustGrants) for all three grant components and a new payment management system.
You can access JustGrants via the Login page or by clicking the “Login” link on the Justice Grants website.
Read the JustGrants announcement for detailed key information, FAQs, and resources. Sign up for JustGrants email updates.
___________________________________________________________________________________________________________
New RePORT and RePORTER Tools
October 13, 2020
Message from NIH's Open Mike
Welcome the New RePORT and RePORTER Tools!
Ten years ago, NIH launched the RePORT (Research Portfolio Online Reporting Tools) website to serve as a one-stop shop for reports, data, and analyses of NIH research activities. Well, drum roll please, a new and modernized RePORT site as well as a faster and easier to use NIH RePORTER have now arrived.
The updated RePORT site strives to meet the needs of today’s users based on feedback received over the years. It is easier, simpler, and quicker to access the same information you have come to rely upon. Right from the homepage, for instance, you can jump into data with interactive charts that connect out to NIH Data Book, RePORTER, and other resources.
Read NIH's Open Mike to see the new NIH RePORTER features.
___________________________________________________________________________________________________________
New NIH Requirement
October 5, 2020
Message from NIH
Required Submission of Financial Conflict of Interest Policy into the eRA Commons Institution Profile (IPF) Module
Effective November 12, 2020, NIH funded recipients will be required to submit their publicly accessible Financial Conflict of Interest policy to NIH via the eRA Commons Institution Profile (IPF) Module (IPF Module). A PDF of the FCOI policy must be submitted by the institutional signing official (SO) via the IPF Module under a new tab labeled, “Policy Documents”.
While the automated requirement goes into effect in November, NIH recognizes that recipients will need to modify their internal systems in order to comply. Therefore, applicants and recipients have until December 1, 2020, to comply with this requirement.
For more details, see the full Guide Notice.
___________________________________________________________________________________________________________
New NIH Requirement
October 1, 2020
Message from NIH
Trainee Diversity Report (RPPR)
Beginning October 30, 2020, the requirement for electronically generated Trainee Diversity Reports will be implemented through the RPPR submission process for institutional research training grants, institutional career development awards, and research education awards through the xTrain system. NIH is requiring institutions to submit RPPRs, Interim Final RPPRs, and Final RPPRs electronically, using the new option available FY2021. Read NOT-OD-20-178 for more information.
___________________________________________________________________________________________________________
NIH Reminder
October 1, 2020
Message from NIH
Reminder: NIH Policy on Use of Hypertext in NIH Grant Applications
The use of hypertext (e.g. hyperlinks and URLS) in NIH applications is restricted due to concerns including reviewer confidentiality, “overstuffing” applications, review consistency, and malware.
There is no change in the NIH policy on the use of hyperlinks. The policy, documented in the NIH SF424 (R&R) Application Guide and a recent reminder Guide Notice, reads:
- Hyperlinks and URLs are only allowed when specifically noted in funding opportunity announcement (FOA) and form field instructions. The use of hyperlinks is typically limited to citing relevant publications in biosketches and publication lists. It is highly unusual for a FOA to allow links in Specific Aims, Research Strategy and other page-limited attachments.
- Hyperlinks and URLs may not be used to provide information necessary to application review.
- Reviewers are instructed against viewing linked sites and are cautioned that they should not directly access a website (unless the link to the site was specifically requested in application instructions) as it could compromise their anonymity and allow for malware to be downloaded onto their computers.
-
When allowed, you must hyperlink the actual URL text so it appears on the page rather than hiding the URL behind a specific word or phrase. Example:
- NIH (http://www.nih.gov/)
Applications that do not follow these instructions, and include unallowable hyperlinks, may be withdrawn from review and funding consideration.
___________________________________________________________________________________________________________
eRA Update
September 23, 2020
Message from eRA Commons
NIH Encourages SOs to Review the Accuracy of Grant Information for FY 2020 Before October 2
As the fiscal year (FY) comes to an end on September 30, 2020, NIH encourages signing officials from recipient organizations to verify the accuracy of their grant assignments to departments or components within their organizations of higher education. Any corrections to the data must be made by 8:00 PM EDT on Friday, October 2, 2020 to be reflected in NIH annual reports. It is imperative that corrections to the data occur before these files are “frozen” to ensure the veracity of NIH’s FY2020 reports.
Background
NIH develops standard reporting files used to produce data found on the RePORT Website, to increase transparency about funded grants, address inquiries from the Department of Health and Human Services, Congress, and the research community, and to fulfill annual reporting requirements on NIH’s expenditures. The data in these files are “frozen” annually to ensure the reporting files produce consistent and meaningful results. One way that RePORT provides information is by school/department; because of inconsistencies in the way information on department and school names are provided in grant applications, grant officials may want to ensure that the information is reflected accurately in NIH systems.
Verifying Your Information
Verify the accuracy of the grant award information for your organization by going to the NIH RePORT Awards by Location and Organization site at https://report.nih.gov/award/index.cfm, selecting FY2020, location and organization. Once the search results are displayed, select the ‘Data’ tab on the far right, click on the Excel Export icon (green) located in the right-hand corner. (See Figure 1). The resulting spreadsheet will provide you the ability to review all the critical information about the award, including the assigned department name.
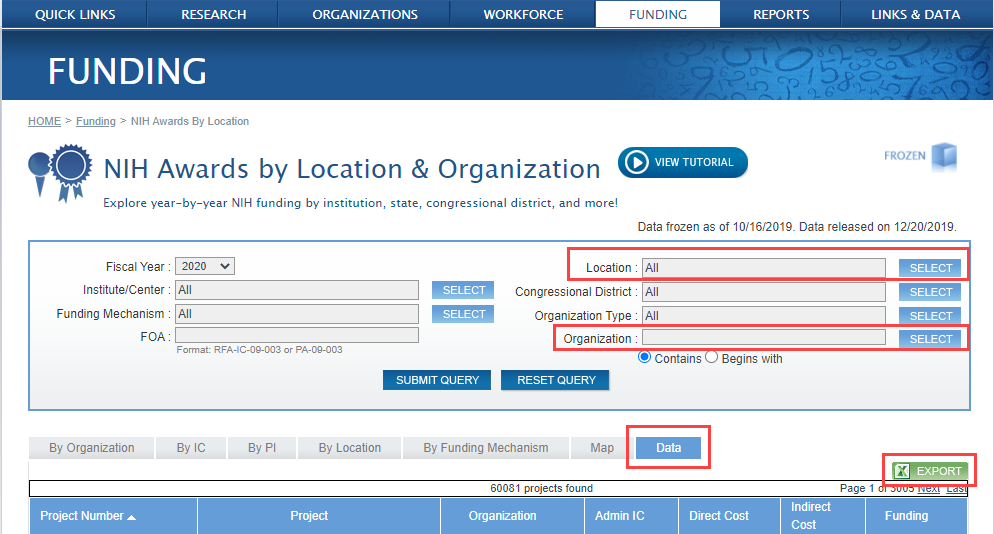 Figure 1: NIH Awards by Location & Organization search screen highlighting the relevant fields (click image to see full size)
Figure 1: NIH Awards by Location & Organization search screen highlighting the relevant fields (click image to see full size)
Reassigning a Grant Within Your Institution
After verifying your award information, if a grant needs to be reassigned within an institution to ensure grant information accuracy, this can be done using eRA Commons’ Re-assign Award feature that is found on the left side of the Status search screen (See Figure 2). Reassignment can only be done by users with the signing official (SO) role within Commons.
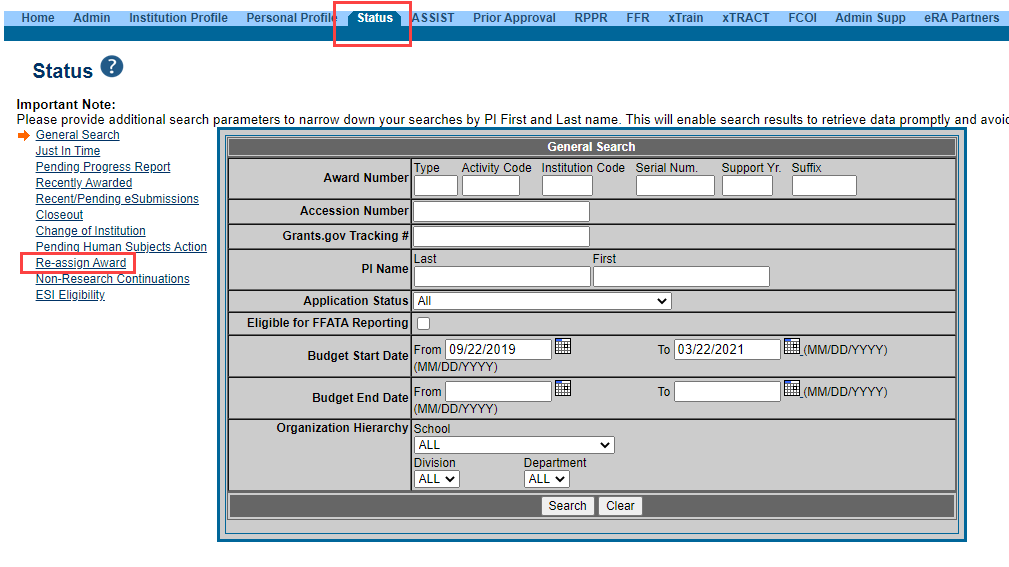 Figure 2: Status search screen showing the Re-assign Award link (click image to see full size)
Figure 2: Status search screen showing the Re-assign Award link (click image to see full size)
The Re-assign Award screen permits the SO to search for awards using a variety of parameters. (See Figure 3) Once the award or awards are selected, the system will guide the SO through the process of reassigning an award.
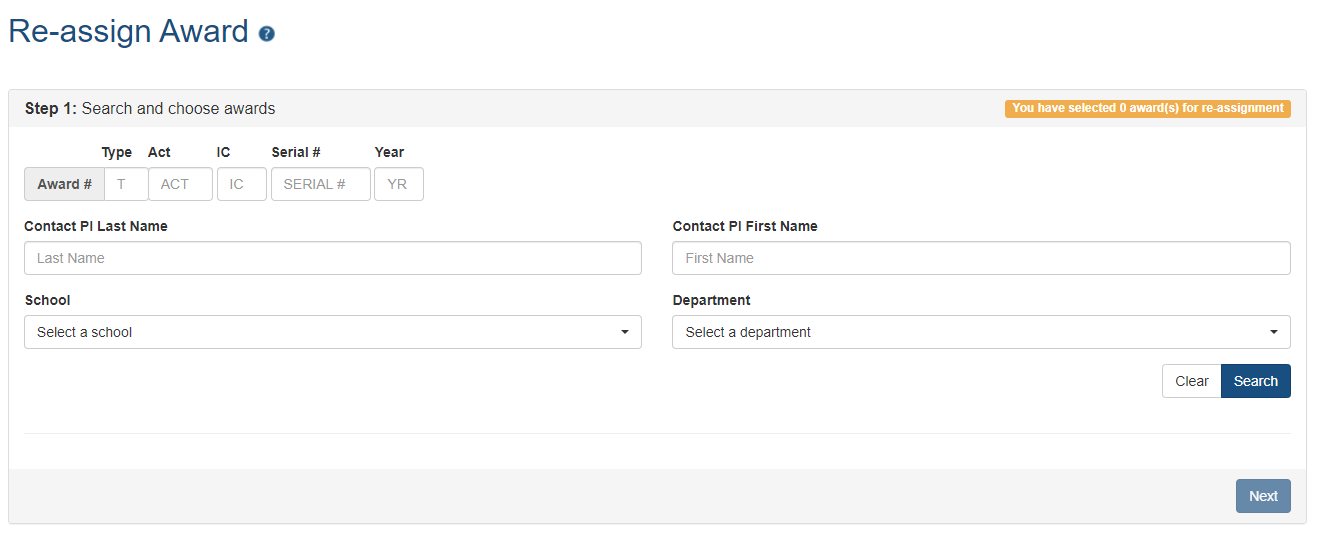 Figure 3: Re-assign Award search screen (click image to see full size)
Figure 3: Re-assign Award search screen (click image to see full size)
For information regarding this information, please refer to Guide Notice NOT-OD-20-175.
For step-by-step instructions on how to reassign a grant, please refer to the eRA Commons online help topic: Steps for SO to Reassign a Grant.
___________________________________________________________________________________________________________
NSF Update
September 22, 2020
Message from NSF
Research.gov Implementation Update
The National Science Foundation (NSF) has been at the forefront in the development of Federal agency electronic systems designed to prepare and submit proposals for Federal financial assistance. From the introduction of FastLane in 1994, to the incremental development of Research.gov as its eventual replacement, NSF has led the way with modern, agile systems tailored to meet the needs of the research community.
While NSF's FastLane system has been a resounding success story, it is now an aging, antiquated system that has become increasingly expensive to maintain and even harder to improve. Over the past few years, NSF has partnered with and received valuable input from the research community, resulting in the development of a modern, flexible Research.gov system that reduces administrative burden to meet the current and future needs of researchers, administrators and organizations. As a result, NSF has successfully migrated important research functions from FastLane to Research.gov including the preparation and submission of annual and final project and outcomes reports, most notifications and requests and award payments. NSF is now taking proactive steps to incrementally move the preparation and submission of all proposals from FastLane to Research.gov with a tentative target date for completion by 2022.
In support of this effort, in the coming weeks and months, NSF will begin making changes to specific funding opportunities to require the use of Research.gov for the preparation and submission of proposals to NSF.1 The Directorate for Biological Sciences (BIO) will soon require the use of Research.gov for the preparation and submission of proposals in response to its core programs that do not have deadline dates. NSF funding opportunities will clearly specify whether submission via Research.gov is available or required.
To ensure that researchers and administrators are prepared for these changes, NSF is developing additional training materials to meet the needs of the community. This includes video tutorials, Frequently Asked Questions, step-by-step guides and a demonstration site. Current training materials are available on the About Research.gov site.
NSF encourages the community to become familiar with Research.gov and to begin using it for the preparation and submission of proposals, as well as to provide NSF with valuable feedback. For additional information, FAQs, opportunities for training and to provide feedback, please visit Research.gov.
Dr. Sethuraman Panchanathan, Director
1During this time, NSF will continue to permit proposals to be prepared and submitted via Grants.gov.
____________________________________________________________________________________________________________
eRA Enhancement
September 1, 2020
Message from eRA News
eRA Enhancement: Ability to Select Awarded Components on RPPR Screen, Instead of Manually Adding for Multi-Project Awards
eRA is pleased to inform you of a new way of selecting components for multi-project awards for the Research Performance Progress Report (RPPR) coming on Thursday, September 3, 2020.
Ability to Select Awarded Components for Multi-Projects
In RPPR, awarded components for multi-project grants will automatically show up on the RPPR Menu screen, reducing the burden on the grantee of individually adding these components. The list of components displayed will be from the last competitive application that was awarded.
Add a New Component (existing functionality)
A grantee can also continue to use the existing feature of adding a new component.
Add the name of the component in the New Component Project Title field, and then from the drop-down menu, select the component type. (See Figure 3 below) Once the new component is added, you can select the edit option from the ellipsis icon (more on this below) and complete the RPPR sections A through H as required.
New Three Dot Menu for Actions
Previously, action options were represented by hyperlinked text in the Action column. This has been replaced by an ellipsis (a three-dot icon). Hover the cursor over the ellipsis and select from the menu options that appear. (See Figure 4 and Figure 5 below).
You can find more information detailed information, including helpful screenshots, in the eRA Enhancement.
____________________________________________________________________________________________________________
NSF Update
August 28, 2020
Message from OSP
Issuance of Proposal Preparation & Award Administration FAQs related to the PAPPG (NSF 20-1)
The National Science Foundation just announced the issuance of a set of Frequently Asked Questions (FAQs) on proposal preparation and award administration related to the NSF Proposal & Award Policies & Procedures Guide (PAPPG) (NSF 20-1).
Some of the topics addressed in the FAQs include conference proposals, cost sharing, deadline dates, indirect costs, international activities/considerations and participant support.
____________________________________________________________________________________________________________
OMB Update
August 21, 2020
Message from [RAD] August 21, 2020
OMB 2020 Compliance Supplement
The Federal Register August 18, 2020 notice announced the release of OBM's 2020 Compliance Supplement for uniform administrative requirements, cost principles, and audit requirements regulations. The 2020 Compliance Supplement replaces the 2019 Compliance Supplement and applies to fiscal year audits beginning after June 30, 2019. According to an OMB memo, the supplement continues implementation of the President’s Management Agenda, Cross Agency Priority (CAP), “Results-Oriented Accountability for Grants,” and includes guidance related to Coronavirus administrative relief. Comments are being accepted until October 10, 2020. Contact GrantsTeam@omb.eop.gov with questions.____________________________________________________________________________________________________________
NSF Update
August 7, 2020
Message from OSP
Updates to NSF Existing Proposal and Award Policy-related guidance
National Science Foundation (NSF) has made updates to the following guidance on their website based on inquiries from the community:- Frequently Asked Questions (FAQs) on NSF’s Implementation of OMB Memorandum M-20-26
- NSF-approved Formats for the Biographical Sketch website
The updates provide clarifications on professional appointments, research endeavors, principal investigators working outside the U.S. for an extended period of time, and reporting on in-kind support and outside consulting activities.
____________________________________________________________________________________________________________
eRA Enhancement
August 5, 2020
Message from eRA News
Change in Format to the Notice of Award Starting October 1, 2020
Starting on October 1, 2020, recipients will see a new standardized Page One of the Notice of Award (NoA). The NoA is the legal document issued to notify the recipient that an award has been made and that funds may be requested from the designated HHS payment system or office. This enhancement is part of HHS’s Reinvent Grants Management initiative to standardize the NoA across various HHS systems and reduce the burden on recipients.
The Department of Health and Human Services (HHS) in collaboration with Operating Divisions (OpDivs) and recipients has developed a standardized Page One of the NoA that will serve as the first page of every HHS NoA for all discretionary awards.
The new format captures key award information for grant recipients in an intuitive and digitally accessible format. For instance, financial award information and federal agency contacts (program official contact information, etc.) will be available on Page One. The remaining sections of the NoA will remain mostly as is, with some data elements moving to Page One from subsequent pages of the NoA.
The new NoA Page One will be used by all HHS OpDivs.
We are developing a number of resources to help recipients get familiar with the new Page One of the NoA. In the coming weeks, please look for these resources on the View Notice of Award page of the public eRA website.
For more information, please see Guide Notice NOT-OD-20-155.
____________________________________________________________________________________________________________
NIH Update
July 31, 2020
Message from NIH
Upcoming Changes to the Notice of Award (NoA) Beginning October 1, 2020
NOT-OD-20-155 announces the upcoming changes to the format of the National Institutes of Health (NIH) Notice of Award (NoA). The Department of Health and Human Services (HHS) has developed a standard Page One to be used across HHS effective October 1, 2020.
The majority of the change will affect the appearance of Page One of the NoA. There will be four sections (see the NOT-OD-20-155 for details):
- Award Data
- Recipient Information
- Federal Agency Information
- Federal Award Information
NIH NoAs will continue to use the following sections, following Page One:
- Section I – Award Data
- Section II – Payment Information
- Section III/IV – Terms and Conditions of Award
In the coming weeks, additional information and samples will be posted on the View Notice of Award webpage.
____________________________________________________________________________________________________________
NSF Dear Colleague Letter
July 30, 2020
Message from OSP
Frequently Asked Questions (FAQs) for NSF 20-087, Dear Colleague Letter
The National Science Foundation (NSF) released FAQs for the May 2020 Dear Colleague Letter (DCL), NSF 20-087. Beginning October 1, 2020, hard deadlines will be removed for Small project proposals submitted to the Core Research Programs in the Directorate for Computer and Information Science and Engineering (CISE). These are CISE divisions for Computing and Communication Foundations (CCF), Computer and Network Systems (CNS), and Information and Intelligent Systems (IIS). Small project proposals may be submitted at any time throughout the year. The Small project proposal allows a budget up to $500,000 and three years.
____________________________________________________________________________________________________________
eRA Information
July 23, 2020
Message from OSP
NIH released news of ASSIST being expanded for submission of administrative supplements:
Beginning Saturday, July 25, 2020, the use of ASSIST for the submission of administrative supplements will be expanded. This release builds on the previous enhancements to ASSIST, outlined in ASSIST Has Been Streamlined to Support Easier Initiation and Submission of Administrative Supplements from April 7, 2020.
As of this date, there will be three methods for initiating an administrative supplement through eRA systems:
- Initiate in ASSIST, enter the Funding Opportunity Announcement (FOA) for an administrative supplement and enter information manually
- Initiate in ASSIST and after entering the Federal ID number of the parent grant award, some of the information from the parent award is prepopulated
- Initiate through eRA Commons and after identifying a specific grant for administrative supplement, be directed by the system to ASSIST where some information from the parent award is prepopulated
For more information, please visit the eRA News webpage.
____________________________________________________________________________________________________________
NSF Update
July 7, 2020
Message from OSP
Significant Changes to Revised Terms and Conditions
National Science Foundation (NSF) has revised several sets of award terms and conditions to align with the PAPPG. There are two significant changes of note outlined in the OSP Blog.
____________________________________________________________________________________________________________
Click to see Proposal Deadline Updates
Don’t Forget to Use FORMS-F
July 1, 2020
Message from NIH Staff
At this point, nearly all grant applications should be using our updated application forms (FORMS-F; NOT-OD-20-026 and NOT-OD-20-077). If you aren’t sure how to tell the form version you are using, check out Do I Have the Right Forms for My Application?
Resources related to form updates:
- How to Apply – Application Guide
- Grants Administration Take 10: NIH FORMS-F Application Forms Update video
- Annotated Form Set for NIH Grant Applications
- Do I Have the Right Form Version For My Application?
- Application Forms, Form Updates, and Choosing the Correct Forms FAQs
There are a few notable exceptions:
-
NIH will accept FORMS-E application form packages for the following FOAs until they are reissued on or around June 25, 2020 (NOT-OD-20-110)
- PA-18-589 Successor-in-Interest (Type 6 Parent Clinical Trial Optional)
- PA-18-590 Change of Grantee Organization (Type 7 Parent Clinical Trial Optional)
- PA-18-591 Administrative Supplements to Existing NIH Grants and Cooperative Agreements (Parent Admin Supp – Clinical Trial Optional)
- PA-18-592 Research Supplements to Promote Re-Entry into Biomedical and Behavioral Research Careers (Admin Supp – Clinical Trial Not Allowed
- PA-18-935 Urgent Competitive Revision to Existing NIH Grants and Cooperative Agreements (Urgent Supplement – Clinical Trial Optional)
-
PA-20-135 Emergency Competitive Revision to Existing NIH Awards (Emergency Supplement – Clinical Trial Optional)
-
Applicants have until June 25, 2020 to complete submission of their in progress administrative supplements to promote diversity using FORMS-E application packages and parent FOAs PA-18-906 or PA-20-166.
- New applications should use FORMS-F application packages and reissued FOA PA-20-222: Research Supplements to Promote Diversity in Health-Related Research (Admin Supp – Clinical Trial Not Allowed).
- A few institutes have issued notices in the NIH Guide extending late application windows for specific opportunities due to COVID-19 impacts (see Late Application Policy Notices section of this page.) Applicants should use FORMS-E application form packages If submitting late applications for due dates on or before May 24, 2020.
Direct questions regarding our form update to:
NIH Office of Policy for Extramural Research Administration (OPERA)
Systems Policy Branch
Email: OPERAsystemspolicy@nih.gov
____________________________________________________________________________________________________________
NSF Guidance OMB Memo
Refer to the guidance on NSF’s implementation of OMB M-20-26 which extends two of the short-term administrative relief from specific requirements contained in 2 CFR Part 200, Uniform Administrative Requirements, Cost Principles and Audit Requirements for Federal Awards. These extensions go beyond what was previously outlined in the OMB M-20-17.
____________________________________________________________________________________________________________
RFI: Rigor of Animal Research
NIH issued a Request for Information (RFI) regarding rigor, transparency, and translatability to improve biomedical and behavioral research involving animal models. Read NOT-OD-20-130 and submit feedback by July 31, 2020.____________________________________________________________________________________________________________
Submission of the FFR
According to NOT-OD-20-127, as of January 1, 2021, NIH grant recipients will be required to submit the SF-425 Federal Financial Report (FFR) in the Payment Management System (PMS) instead of eRA Commons.
____________________________________________________________________________________________________________
Impact Scores & Summary Statements
June 18, 2020
Message from NIH Staff
Effective June 24, 2020, summary statements and overall impact scores for grant applications will be available on the eRA Commons Status Information screen to users with the signing official (SO) role. Currently, only principal investigators can view the scores and summary statements.
This change came about following the update to the System of Records Notice (SORN) for eRA records, allowing NIH to disclose information to applicant organizations for the purpose of communicating about matters related to agency award programs.
This means that the NIH will make the overall impact score and all current and previously issued summary statements available to the applicant organization through the NIH eRA Commons. The user must have the signing official (SO) role in the eRA Commons in order to view the impact score and the summary statement.
For help accessing the Status Information screen, please see the online help for the Status Module. Once the system is released on June 24, there will be a help page with specific instructions for how SOs can access review outcomes.
For information regarding this change, please refer to Guide Notice NOT-OD-20-126.
____________________________________________________________________________________________________________
New “All About Grants” Podcast
Message from NIH Staff
Yes, we are talking about contracts in this next installment of the
NIH’s All About Grants podcast series. Our guests will be George Kennedy and Brian O’Laughlin, who are acquisition staff from the National Institute of Allergy and Infectious Diseases and National Institute on Drug Abuse, respectively. The conversation (MP3 / Transcript) will introduce you to the world of contracts at NIH, what they are, how they differ from grants, where to find them, what types of research are solicited, tidbits to focus on when developing a proposal, and more.
Check out the System for Awards Management (SAM) to find more information on contract solicitations.
____________________________________________________________________________________________________________
Change in Status Including Absence of Key Personnel
NOT-OD-20-124 clarifies expectations regarding change in status, including absence of Program Directors, Principal Investigators, and other key personnel named in the Notice of Award. NIH expects that funded research occurs within an environment that is safe, healthful, and conducive to high-quality work. Refer to modified guidance,"Change in PD/PI and Other Senior/Key Personnel," NIH GPS Section 8.1.2.6. For more information read this week's post, New Steps to Help Ensure Safe Work Environments for NIH-Supported. Research.
____________________________________________________________________________________________________________
NIH Reissue of Parent Announcements
According to NOT-OD-20-110, the reissue of Parent Announcements and the urgent/emergency competitive revision of Funding Opportunity Announcements is in progress. Applicants should continue using the Current Announcements/Forms Packages until NIH reissues, which is expected by June 25, 2020. Changes apply to NIH grant application forms/instructions due on or after May 25, 2020 (FORMS-F).
____________________________________________________________________________________________________________
A Walk-Through of the PHS Human Subjects & Clinical Trials Information Form
June 2, 2020
Message from NIH Staff
We’ve updated the Walk-through of the PHS Human Subjects & Clinical Trials Information Form video to align with our latest application form update (FORMS-F). In just six minutes, you’ll learn how to use the form and how to complete both delayed onset and full study records. The video describes each of the five sections of a study record and points out which fields are required for human subjects and clinical trial studies. You might even discover a handy tip or two along the way.
For a summary of significant form changes and detailed guidance for completing the form, check out our FORMS-F application instructions.