Below, you'll find the latest sponsor updates, guidance, information, and resources regarding COVID-19. Also, please see the latest COVID-19 information from the NIH and OSP.
______________________________________________________
New NIH COVID-19 Update
February 14, 2022
Message from NIH
Are COVID-19 Flexibilities Related to Animal Care and Use Still in Effect?
The flexibilities allowed by the NIH Office of Laboratory Animal Welfare are continuing due to the recent surges in the COVID-19 pandemic throughout the country and the impact on institutions’ operations.
Additional resources are available on OLAW’s COVID-19 Pandemic Contingency Planning webpage to assist Assured institutions in preparing for and coping with the current COVID-19 pandemic while maintaining compliance and good animal welfare. For more information, read the full NIH post.
______________________________________________________
New NIH COVID-19 Update
February 8, 2021
Message from NIH
Extensions for Early Career Scientists Whose Career Trajectories Have Been Significantly Impacted by COVID-19
The COVID-19 pandemic, along with extensive mitigation measures, has adversely affected progress in many biomedical research settings. NIH issued a Guide Notice last week detailing their approach to support early career scientists whose career trajectories may have been significantly affected by the pandemic. Specifically, NIH is providing an opportunity for recipients in their last year of NIH Fellowship (“F”) and NIH Career Development (“K”) awards who have been impacted by COVID-19 to request extensions. Such extensions will be considered on a case-by-case basis, within the existing constraints of available funding.
For more information, please read the full NIH article.
______________________________________________________
New NIH COVID-19 Update
January 28, 2021
Message from NIH
How Do I Submit Preliminary Data as Post-Submission Material Under the Special Exception During the COVID-19 Pandemic?
You may submit data as post-submission material under the special exception for the COVID-19 pandemic if:
- The timing is after submission of your grant application but before peer review. Unless stated otherwise in the Funding Opportunity Announcement, the deadline for post-submission materials is 30 days before the review meeting.
- The data material is not intended to correct oversights or errors discovered after submission of the application.
- The Funding Opportunity Announcement (FOA) used for submission allowed preliminary data in the application. If this applies to you, see NIH Will Continue to Accept Preliminary Data as Post-Submission Material Through August/October 2021 Councils for more details, including deadlines.
For videos, please follow the directions in NOT-OD-18-093 and NOT-OD-20-061.
Remember:
- You must demonstrate concurrence of your Authorized Organization Representative.
- You must send the post-submission materials directly to the Scientific Review Officer who is managing the review of your application.
For step-by-step directions for all other materials, please refer to our previous post, “How Do I Submit Post-Submission Materials for My Application?”
Still have questions? Please reach out to NIH for assistance.
______________________________________________________
New NIH COVID-19 Website
January 19, 2021
Message from NIH
Check Out NIH’s New COVID-19 Research Website
We are pleased to announce that the new NIH COVID-19 website launched last week. The site provides a central location for trusted, up-to-date, accurate information about NIH research and our strategic role in COVID-19 research. The site complements information made available on our COVID-19: Information for NIH Applicants and Recipients of NIH Funding webpage.
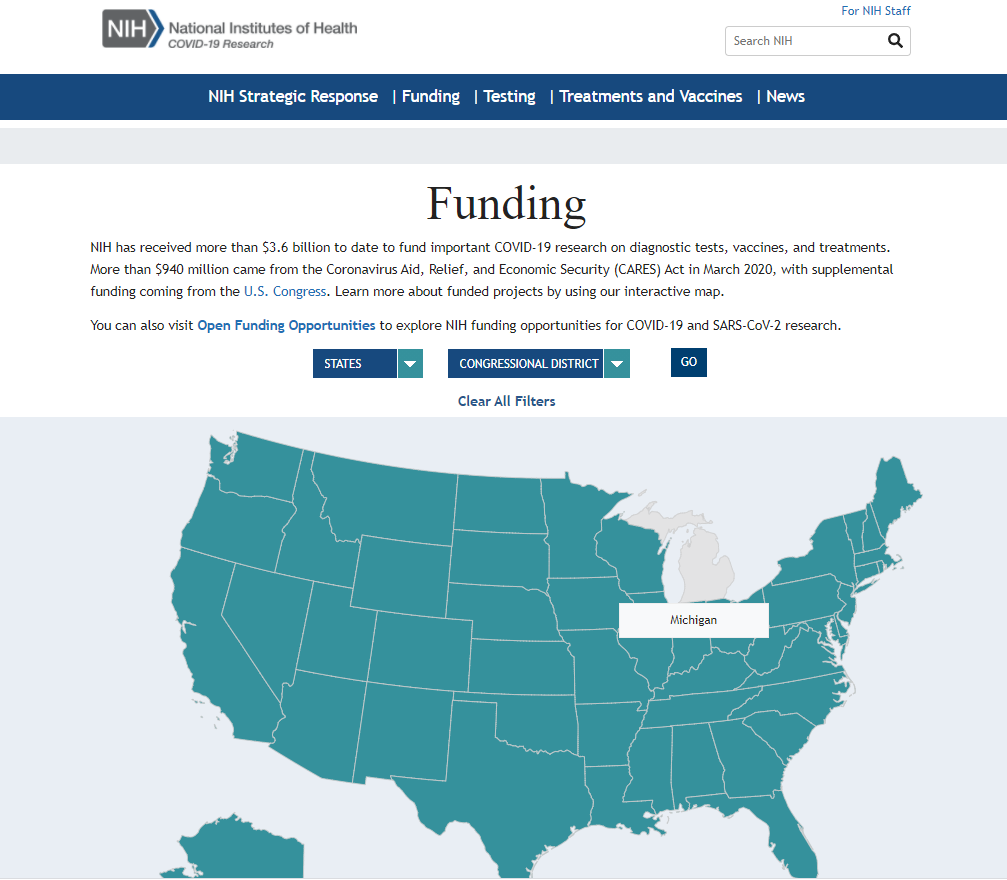
The new site includes information about key programs such as the Accelerating COVID-19 Therapeutic Interventions and Vaccines public-private partnership and the Rapid Acceleration of Diagnostics initiative to develop state-of-the-science diagnostic tests for COVID-19. Users are also able to search information on funded research by state, institution, Congressional district, and more.
To support ongoing efforts to direct the public to critical information on COVID-19, the website also includes links to information on:
- Vaccines, treatments, and testing
- Clinical trials and how to participate
- How to donate plasma
Resources from NIH’s Community Engagement Alliance Against COVID-19 Disparities and Federal agencies such as the Centers for Disease Control and Prevention and Department of State
______________________________________________________
New NIH FAQs
December 2, 2020
Message from NIH
New FAQs on Policy for Charging PPE to NIH Grants
FAQs now available to clarify our recent guidance on the ability to direct charge personal protective equipment (PPE) costs to clinical trials and clinical research awards (NOT-OD-20-164).
1. What is the effective date of Guide notice NOT-OD-20-164?
Guide notice NOT-OD-20-164 is effective for awards issued on or after the date of the notice, September 11, 2020.
2. Is this retroactive at all? For example, is there an issue if a PI purchased PPE in excess of $500K last month?
This policy applies effective the date of the Guide Notice, which was September 11, 2020. It is not retroactive.
3. The policy states that this guidance does not apply to grants or cooperative agreements that are not conducting clinical trials/clinical research. Can recipients that are not conducting CT or CR direct charge PPE?
Recipients conducting research that is not a clinical trial or clinical research may charge PPE as a direct cost only when such charges align with their negotiated indirect cost rate agreement. Recipients may not charge PPE as a direct cost if their negotiated rate includes PPE costs, and any direct charges must be in direct support of and allocable to the NIH grant.
4. Can PPE be charged to grants supporting biosafety labs such as BSL-3 and BSL-4 facilities?
Yes, PPE can be charged as a direct cost, when such charges align with the negotiated indirect cost rate agreement, and the charges are in direct support of and are allocable to the grant.
5. Can foreign recipients charge PPE as a direct?
Foreign recipients may charge PPE as a direct cost when such charges are in direct support of and are allocable to the grant.
6. Is the $500,000 direct cost limit cumulative per budget period, or over the project period?
The $500,000 limit applies to each budget period. Expenses for PPE may total up to $500,000 per budget period.
7. Does the $500,000 limit per budget period include all costs for the parent and any subawards?
Yes, the $500,000 limit includes the parent award as well as any subawards or supplements.
8. Is there a standard definition of Clinical Research?
Yes, the NIH Grants Policy Statement defines Clinical Research as:
Research with human subjects that is:
1) Patient-oriented research. Research conducted with human subjects (or on material of human origin such as tissues, specimens, and cognitive phenomena) for which an investigator (or colleague) directly interacts with human subjects. Excluded from this definition are in vitro studies that utilize human tissues that cannot be linked to a living individual. It includes: (a) mechanisms of human disease, (b), therapeutic interventions, (c) clinical trials, or (d) development of new technologies.
2) Epidemiological and behavioral studies.
3) Outcomes research and health services research
Studies falling under 45 CFR 46.101(b) (4) (Exemption 4) are not considered clinical research by this definition.
______________________________________________________
NIH's Extended Guidance
December 1, 2020
Message from NIH
Extended Guidance for Preparing Applications During the COVID-19 Pandemic
NIH grant applications should NOT include contingency plans that would outline steps needed to recover from temporary, emergency situations, or institutional return-to-the-workplace plans, resulting from the COVID-19 pandemic.
Contingency plans will not be considered in peer review but, if needed, COVID-19 contingency plans will be requested and carefully considered by NIH staff before funding.
Reviewers will continue to receive instruction to assume that temporary, emergency problems arising from the COVID-19 pandemic will be resolved and complications related to COVID-19 should not affect their scores. Reviewers will be instructed to disregard situations due to the COVID-19 pandemic, e.g., temporary declines in productivity, availability of key personnel, proposed patient populations, animal facility shutdowns, etc.
This guidance has been extended until further notice, as announced in NOT-OD-21-026.
______________________________________________________
NIH Seeking Researchers' Perspective
October 29, 2020
Message from NIH
Researchers, if You Received a Survey, Please Provide Us with Your Perspective on the Impact of COVID-19
Since March, COVID-19 has greatly impacted the way we all work. NIH has been tracking how well our policies meet the needs of our research community in response to the ongoing pandemic. To get a better understanding of how COVID-19 is impacting our extramural researchers, we launched the Impact of COVID-19 on Extramural Researchers Survey in mid-October. If you received an invitation to take this survey, please take 15-20 minutes to complete it. This survey will be open until Friday, November 13th. The results from the survey will inform policy and program decisions, so participation is critical.
Thousands of you have responded, and we thank you very much for your participation. If you were invited to do so and have not yet participated, we encourage you to do so at your earliest convenience. You will have received an invitation if you are part of the sample.
The survey is confidential and run by a third party, who will share only de-identified survey data with NIH. Survey results will be analyzed and reported to leadership in aggregate.
Thank you for your continued support of this effort. Please don’t hesitate to reach out to jennifer.miller-gonzalez@nih.gov if you have questions about this effort or the survey itself.
___________________________________________________________
NIH is Seeking Your Ideas
October 29, 2020
Message from NIH
Seeking Your Ideas on the NIH-Wide Strategic Plan for COVID-19 Research
In less than a year, we have learned much about SARS-CoV-2 and COVID-19 disease. The NIH-Wide Strategic Plan for COVID-19 Research, released last July, has helped us get to this point. The Plan prioritizes conducting fundamental research; advancing diagnostics, treatments and prevention strategies; and redressing poor COVID-19 outcomes in health disparity and vulnerable populations. Cutting across all of these priorities is an emphasis on the importance of scientific collaboration, the research workforce, and data science as keys to the response.
From shifting public health needs to the unprecedented pace of biomedical discovery, everything about the coronavirus response is evolving. This goes for the plan as well, so too must it evolve.
We want your help on the next iteration of the Plan. A Request For Information released yesterday seeks public feedback on the current Plan (NOT-OD-21-018). You or your organization can submit ideas here by December 7, 2020.
If you have noted significant research gaps or barriers in the original Plan, let us know. Or perhaps you can share new resources that NIH can leverage to advance one of the plan’s priorities. Maybe a new scientific technique has emerged that could revolutionize COVID-19 research, well send the suggestion our way. We look forward to receiving your thoughts on ways we can continue tackling coronavirus disease going forward.
______________________________________________________
New RPPR/Sponsor Guidance
July 24, 2020
Message from [RAD] July 24, 2020
Two guidance documents were released to support researchers in reporting impacts of COVID-19. Review the NIH RPPR and the Sponsored Guidance COVID-19 Impact Statement. Visit the OSP website for University COVID-related guidance, FAQs and resources.
______________________________________________________
Roundup of New COVID-19 Resources for NIH Applicants & Recipients: Part 3
July 30, 2020
Message from NIH Staff
We continue to add new resources to our COVID-19: Information for NIH Applicants and Recipients of NIH Funding webpage. We hope they are helpful in navigating this unprecedented situation. Here is a summary of what’s new since the last Nexus:
- Updated infographic describing the peer review process during COVID-19 highlights policies in effect for the upcoming round of due dates
- Link to new dedicated page for funding opportunities specific to COVID-19 provides visibility into expiration dates and separates active vs expired FOAs
- New section on Funded Grants provides the ability to view COVID-19 related grant funding. Explore funding further using the COVID-19 Response search filters in RePORTER (under “Additional Filters”)
- All FAQs revised to align with NIH implementation of OMB memo M-20-26
- Updated animal welfare FAQs
- Updated overview presentation and talking points, for quick summaries of flexibilities
We know it can be a challenge to track new information as it becomes available. We are noting changes to the website in the page update history, tweeting from @NIHgrants as things get posted, and we will continue to highlight new resources in the Nexus.
______________________________________________________
NIH RPPR Affected by COVID-19
July 17, 2020
Info from OSP FAQs for COVID-19 - Sponsored Guidance
If the progress of my NIH project has been affected by COVID-19, how should I share this information with NIH?
NIH released an updated FAQ (section IV, question 1 of the FAQ), requiring PI’s to report COVID impacts via their RPPR rather than through a letter to their Program Officer. PI’s should only include impact statements on awards that have been impacted due to COVID and address relevant issues of scientific progress. This guidance is specific to NIH and the expectation is that we are still monitoring all awards for COVID impacts as we don’t yet know how other agencies will expect communication on COVID impacts. Please reach out to OSP or your school research administration office with any questions.
______________________________________________________
Updated CDC Flexibilities Guidance
July 10, 2020
Message from the CDC
CDC recently updated its flexibilities guidance for recipients and applicants of federal financial assistance in accordance with OMB Memorandum M-20-26.
The guidance outlines:
- the flexibilities from M-20-11 still in effect through July 26, 2020, for awards supporting research and services necessary to carry out the emergency response related to COVID-19,
- the two flexibilities from M-20-26 which apply to an expanded group of recipients impacted by COVID-19, and
- the expired flexibilities from M-20-17.
Although some of the flexibilities provided through OMB have expired and more could in the coming months, CDC continues to have the ability to issue exceptions on a case-by-case basis in accordance with 45 CFR § 75.102. If a recipient anticipates needing any of the flexibilities, they should contact their assigned grants management specialist/program official.
As OMB or HHS provide any additional flexibilities and/or guidance, CDC will update its guidance accordingly. CDC remains committed to working closely with recipients to reduce administrative burden where needed due to the COVID-19 pandemic.
COVID-19 Affecting Federal Agencies
The Federal Demonstration Partnership (FDP) met virtually May 21 to May 28, 2020. Videos and slides from the seven sessions are available on the FDP website. In addition to scheduled updates, sessions included a variety of perspectives on how COVID-19 is affecting federal agencies, universities, and the overall research enterprise. Specific topics included: updates from federal agencies; a presentation on strategic responses to COVID-19 by NIH’s Dr. Mike Lauer, followed by agency-specific updates on COVID-19 from NIH, NSF, and DoD; faculty and administrator perspectives concerning COVID-19’s impact on research at universities; COVID-19 and Data Transfer and Use Agreements; Subawards; and standardizing the IACUC animal protocol template. The next FDP meeting is September 2020. Questions? Contact Melissa Korf, Melissa Maher, or Kevin Ritchie, HMS Office of Research Administration.
______________________________________________________
NIH Late Applications
June 16,2020
Message from NIH Staff
NIH has announced an updated late policy for the parent institutional training grants which have just a single due date each year. In addition, some NIH ICs have issued late notices for specific funding opportunities, which are posted on the Coronavirus Disease 2019 (COVID-19): Information for NIH Applicants and Recipients of NIH Funding website. For all other Funding Opportunity Announcements NIH is taking a very flexible stance for applications submitted within the standard two week late policy. Applicants should include a cover letter with an explanation for the late submission.
Find this and more frequently asked questions on our COVID-19 Flexibilities FAQ page.
______________________________________________________
NIH Post-Submission Material Policy
June 9, 2020
Message from Mike Lauer
According to Dr. Michael Lauer, NIH Deputy Director Extramural Research, COVID-19 mitigation measures have adversely affected the ability of many researchers to generate preliminary data. "Now that all 50 states have begun to reopen, investigators may be better positioned to develop preliminary data. We want to give them the opportunity to have that data considered for this application submission round," Open Mike blog. Read NOT-OD-20-123 for information on NIH, AHRQ, and NIOSH accepting a one-page update with preliminary data as post-submission materials for applications.
______________________________________________________
NIH COVID-19 Diagnostics Innovation Initiative
April 29, 2020
Message from NIH Staff
NIH launched a new initiative aimed at speeding innovation, development and commercialization of (COVID-19) testing technologies. The Rapid Acceleration of Diagnostics (RADx) initiative will infuse funding ($1.5 billion) into early innovative technologies to speed up the development of rapid and accessible testing. For detailed information, please visit the NIH's website.
______________________________________________________
NIH Temporary, Emergency Situations Due to COVID-19
April 21, 2020
Message from Sally Amero
As we continue to address the effects of the COVID-19 public health emergency on NIH-supported research, we are aware of applicant concerns about the potential impact of this temporary emergency situation on the outcome of peer review. We want to reassure applicants that we released guidance for reviewers that makes it clear that, when reviewing applications during the coronavirus pandemic national emergency, reviewers should assume that issues resulting from the coronavirus pandemic, such as the following, should not affect scores.
- Some key personnel on grant applications may be called up to serve in patient testing or patient care roles, diverting effort from the proposed research
- Feasibility of the proposed approach may be affected, for example if direct patient contact is required
- The environment may not be functional or accessible
- Additional human subjects protections may be in order, for example if the application was submitted prior to the viral outbreak
- Animal welfare may be affected, if institutions are closed temporarily
- Biohazards may include insufficient protections for research personnel
- Recruitment plans and inclusion plans may be delayed, if certain patient populations are affected by the viral outbreak
- Travel for key personnel or trainees to attend scientific conferences, meetings of consortium leadership, etc., may be postponed temporarily
- Curricula proposed in training grant applications may have to be converted to online formats temporarily
- Conferences proposed in R13/U13 applications may be cancelled or postponed.
NIH will work with the applicant to resolve issues related to temporary, emergency conditions prior to award.
We have also had many questions from applicants asking what they should do if they don’t have enough preliminary data for the application they had planned to submit. While it may not be the most popular answer, we always recommend that applicants submit the best application possible. If preliminary data is lacking, consider waiting to submit a stronger application for a later due date.
The COVID-19 reviewer guidance, along with FAQs for applicants and awardees can be found in our central repository of resources, Coronavirus Disease 2019 (COVID-19): Information for NIH Applicants and Recipients of NIH Funding.
______________________________________________________
Roundup of New NIH COVID-19 Resources: Part 2
April 17, 2020
Message from NIH Staff
We continue to add new resources to our COVID-19: Information for NIH Applicants and Recipients of NIH Funding webpage. We hope they are helpful in helping you navigate this unprecedented situation. Here is a summary of what’s new since the last Nexus:
- Many new and updated FAQs on training grants and career development awards, certificates of confidentiality, and registration requirements for application submission. A listing of specific FAQ changes can be found on our page update history.
- New funding opportunities specific to COVID-19
- New NIH Resource to Analyze COVID-19 Literature: The COVID-19 Portfolio Tool
We know it can be a challenge to track new information as it becomes available. We are noting changes to the website in the page update history, tweeting from @NIHgrants as things get posted, and we will continue to highlight new resources in the Nexus.
______________________________________________________
NIH Post-submission Revisions due to COVID-19
April 17, 2020
Message from NIH Staff
Institutions affected by COVID-19 will be allowed to submit post-submission grant application materials to revise information that was submitted in an application as long as the materials are received at least fourteen days before the start of the review meeting.
The post-submission grant application materials policy remains in effect. Only the types of materials allowed under the policy can be accepted.
A letter of explanation (maximum of one page) is required.
Find this and more frequently asked questions on our COVID-19 Flexibilities FAQ page.
______________________________________________________
Cyber Safety & COVID-19
April 17, 2020
Message from NIH Cyber Safety Campaign Team
The current outbreak of the novel coronavirus (COVID-19) has introduced new cybersecurity risks both at NIH and across the globe. As targeted phishing attacks prey on our desire to access trustworthy information and many of us make a shift toward remote work, we all need to be vigilant and take accountability for cyber safety.
Be Vigilant – Protect Against Phishing Attacks
Phishing attacks related to COVID-19 are on the rise. Over the past few weeks, several federal agencies and international organizations, including the World Health Organization, have issued cybersecurity alerts about criminal groups who are exploiting the pandemic for their own gain. INTERPOL also issued a targeted warning to hospitals and healthcare institutions at the forefront of the COVID-19 response about ransomware attacks that, “are designed to lock them out of their critical systems in an attempt to extort payments.”
In order to mitigate the risk of these attacks, we all need to know how to recognize and report phishing messages, which can serve as a gateway for malicious actors to enter our systems. Here are a few tips:
- Know how to report phishing messages in your inbox. Phishing emails are real and can show up in our inboxes at any time. That’s why we all have to feel comfortable identifying and reporting them using the “Report Phishing” button in Outlook (or the equivalent feature in another email system) if a message seems suspicious.
- Get your information only from trusted sources. When looking for updates on COVID-19, refer to websites of trusted organizations such as the Centers for Disease Control and Prevention (CDC). Remember that a public health organization will never send you an email asking for log-in credentials, a Social Security number, or payment details in exchange for access to information.
- Think before you click. Make it a habit to carefully inspect all emails to verify their validity before you download any attachments or click on embedded links. Be especially watchful for invasive and aggressive advertising, which may be a ploy to frighten you into acting quickly without thinking. Always verify that the sender’s full email address, including the domain after the “@” symbol, is correct.
Be Secure – Protect Your Home Office
Just as we can take steps to reduce our risk of contracting or spreading COVID-19, there are also steps that each of us can take to reduce the risk of a cybersecurity breach while working remote. To protect yourself and your organization, pay special attention to the following remote work cyber-safety precautions:
- Protect your network. Enable the stronger WPA2 type of encryption on your home router by using the router’s IP address to access its configuration page. If you have not already done so, you should also change your router’s default password to one that satisfies strong password guidelines.
- Secure your equipment. Never let friends or family use your work laptop, phone, or other equipment. Select a designated area within your home from which to work so that you can limit access to your files and computer. Make sure that you are using a screen lock with a strong password whenever you leave your computer unattended, and don’t leave your computer or phone anywhere where they could be visible from outside your house.
- Don’t do work from unsecured personal devices. Any personal devices used to access work files or perform work activities should be protected with your organization’s mobile device management solution. Never use a cable to connect your unsecured personal phone to your work laptop, even just to charge it or move pictures between devices.
- Connect securely. Avoid connecting to free public Wi-Fi. If you do not have access to secure Wi-Fi while working outside your home, use your phone’s mobile hotspot to connect, tethering your laptop as needed. Use VPN to access your organization’s network while working remotely.
- Safeguard sensitive information. Lock confidential paper documents in a secure cabinet inside your home office. When you are ready to dispose of paper documents containing sensitive information, keep them safe until you can bring them to your office to shred them securely. Remember to enable encryption when sending sensitive information by email.
- Don’t share log-in credentials. One of the most important ways we can safeguard our research is by never sharing log-in credentials or passwords. Sharing credentials puts NIH and your organization’s security at risk by potentially exposing sensitive information to unauthorized individuals. If an individual needs access to a specific system, they must go through the proper channels to be authorized and receive their own unique credentials.
- Use your organization’s approved tools to conduct secure virtual meetings. Check with your IT staff to verify which virtual tools are cleared for use by your organization. When using virtual meeting tools, be sure to identify yourself when you sign in and before speaking so that others know who you are. For more information on conducting secure virtual meetings, please see the National Institute of Standards and Technology’s (NIST) helpful guidance on securing virtual meetings.
Be Responsible – Report Any Concerns
If you are involved in a cybersecurity incident of any kind, such as clicking a potentially malicious link in an email, losing your work laptop, or receiving an unencrypted email containing sensitive information, you must immediately report the incident to your organization’s IT security authority.
Be Prepared – Learn More About Cyber Safety
Cybersecurity is constantly evolving in the face of increasingly aggressive and sophisticated threats. To continue protecting ourselves, our organizations, and our research; we should all be continuously learning about these emerging cybersecurity threats. To learn more now, please see the resources below which provide additional guidance from various federal agencies on cybersecurity risks related to COVID-19:
- CDC – COVID-19-Related Phone Scams and Phishing Attacks
- DHS/CISA – UK And US Security Agencies Issue COVID-19 Cyber Threat Update
- FCC – COVID-19 Consumer Warnings and Safety Tips
- DOJ – Combatting Coronavirus Fraud
- For NIH internal users, we invite you to access the NIH Cyber Safety Awareness Campaign website, a great place to find easy-to-understand resources on how to stay cyber safe.
The bottom line is that cyber risks are closer to us than we might expect. We may feel that cyber safety protocols are about compliance for the sake of compliance, but the reality is that cyber safety is about protecting our people and our science. Now more than ever, we all have a responsibility to safeguard ourselves, our organizations, and our research by making cyber safety a priority in our daily work.
______________________________________________________
Security of NIH Virtual Peer Review Meetings
April 16, 2020
Message from Dipak Bhattacharyya
Guest post by Dipak Bhattacharyya, Chief Information Officer of the NIH Center for Scientific Review (CSR), originally released on the Review Matters blog
CSR will conduct all summer peer review meetings using one of three platforms – 1) video; 2) telephone; 3) web-based discussion. A majority will take place using the Zoom video platform. We want to provide information about how we are maintaining the security and confidentiality of our review meetings.
The Zoom video platform that we are using is not the same as that used by schools or by you at home. Instead, we are using a FedRAMP-certified version of Zoom within the zoomgov.com domain. It meets requirements for other agencies that handle very sensitive information, including the Department of Homeland Security. FedRAMP certification means, for reviewers, the platform can be used without risking installation of malware and, for applicants, meetings remain confidential. Key features include:
– All video traffic is highly encrypted and continuously monitored via stringent security controls in place
– Strong configuration management is in place to prevent any unauthorized change of system
– All video traffic is managed by a U.S.-based company (Amazon Web Services government cloud) and stays in the U.S.
To ensure confidentiality of review meetings, we’ve imposed additional security settings that limit meeting attendance to reviewers and NIH staff and prevent recording of information. No one can attend a CSR Zoom meeting without invitation and vetting. Settings CSR has imposed include:
– Enabling passwords
– Requiring confirmation of identity of meeting participants
– Disabling recording, screen sharing, livestreaming, autosaving of chats
For more general information on cyber security at NIH, see the April 8 2020 Open Mike post.
______________________________________________________
New NIH Resource to Analyze COVID-19 Literature
April 15, 2020
Message from George Santangelo
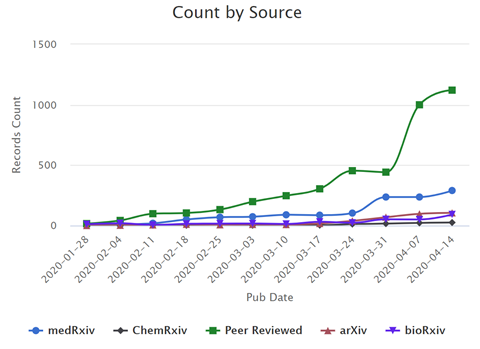
In the past few months, the scientific community has ramped up research in response to the SARS‑CoV‑2 pandemic; dozens of peer-reviewed articles and preprints on this topic are being added to the literature every day (Figure 1). This rapidly expanding effort has created a need for a human-curated resource to explore and analyze advances in SARS‑CoV‑2/COVID-19 research as they accumulate in real time. To address this need, the NIH Office of Portfolio Analysis (OPA) has assembled a comprehensive portfolio of COVID‑19 publications and preprints that is freely available to the public. The COVID-19 portfolio is updated daily with new literature selected for inclusion by subject matter experts.
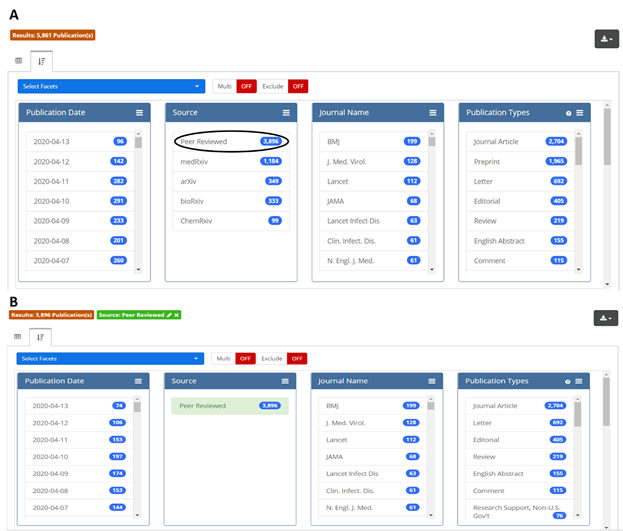
This new resource is designed to provide maximum flexibility and ease-of-use for researchers. Users can take advantage of a full spectrum of Boolean, proximity, and other search methods to query full text and all available supplemental data, or they can limit their search to specific fields, including abstract, author affiliation, or last author. They can also drill down on data once a search is completed. Figure 2 shows a simple example: if a user is interested in analyzing only the peer-reviewed subset of search results, a single click on the “Peer Reviewed” option in the Source facet (circled in black, panel A) will return the desired results (panel B). Some facetable fields, including journal, article type, and author affiliation, are derived from the underlying source data; others, including chemicals & drugs, conditions, and targets, were generated by OPA machine learning/artificial intelligence methods. All data is downloadable as a CSV or Excel file and includes direct links to the publications and preprints. Also, all searches generate stable URLs that users can share with each other.
The tool also includes a visualization feature that groups articles into clusters based on key terms. This allows users to obtain, at a glance, the topic areas returned by their search. The clusters in the visualization are also interactive, which allows narrowing of the results to focus on a specific topic of interest. As an example, Figure 3 shows the results of a search of titles, abstracts, full text, and supplemental text with the terms “protease inhibitor” AND ritonavir (search done at 5 pm 4/14/2020); note that a plurality of the results of this search can be found on the ChemRxiv preprint server. Both the visualization image and results can be exported for downstream applications.
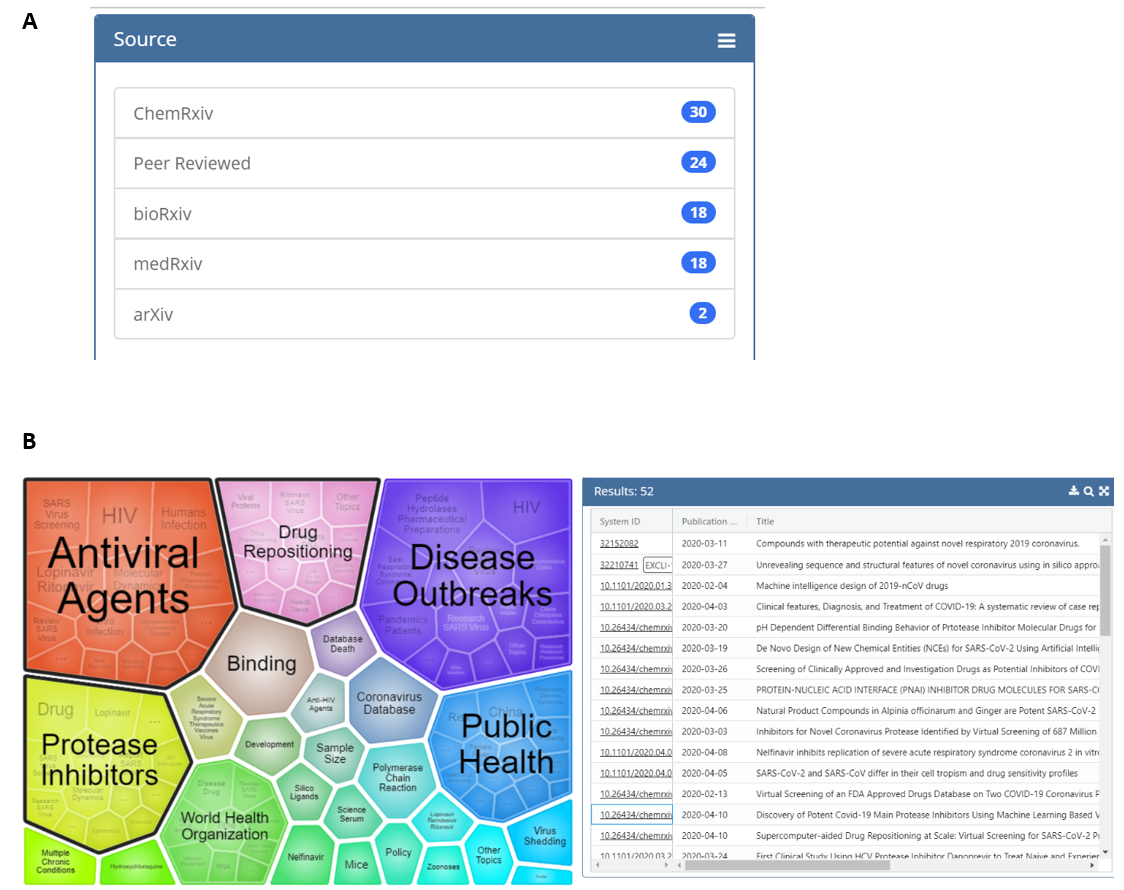
We invite you to explore this resource and are excited to see how the research community will use it to gain insight into the COVID-19 outbreak. OPA will continue to add publication sources and features to support the needs of users. Comments are welcome and can be provided directly through the Feedback button at the bottom left of the browser in the tool.
______________________________________________________
Roundup of New NIH COVID-19 Resources
April 13, 2020
Message from NIH Staff
We continue to add new resources to our COVID-19: Information for NIH Applicants and Recipients of NIH Funding webpage. We hope they are helpful in helping you navigate this unprecedented situation. Here is a summary of what’s new since the last Nexus:
- An overview of information for NIH applicants and recipients related to COVID-19 (available in PowerPoint and Word document formats)
- Many new and updated FAQs on foreign components, animal welfare, continuous submission, timing of reference letters, salaries, donating PPE, prior approval, contracts, and training, fellowship and career development awards. A listing of specific FAQ changes can be found on our page update history.
- A link to a new webpage, COVID-19 Pandemic Contingency Planning for Animal Care and Use Programs
- A link to a webinar, COVID-19 Pandemic Response Resources and FAQs for Animal Care and Use Programs
- A link to OHRP Guidance on COVID-19
- A link to Virtual NIH Activities for Trainees Outside the NIH
- New funding opportunities specific to COVID-19
We know it can be a challenge to track new information as it becomes available. We are noting changes to the website in the page update history, tweeting from @NIHgrants as things get posted, and we will continue to highlight new resources in the Nexus.
______________________________________________________
NIH COVID-19 Funding and Funding Opportunities
April 13, 2020
Message from Mike Lauer
As you can imagine, NIH is devoting significant resources to COVID-19. In addition to dedicating regularly appropriated funds, to date NIH has received emergency funding for COVID-19-related activities in two supplemental bills (available from the NIH Office of Budget website), that together provide:
- $1.532 billion for NIAID
- $103.4 million for NHLBI
- $60 million for NIBIB
- $36 million for NCATS
- $30 million for the NIH Office of Director
- $10 million for NIEHS
- $10 million for NLM
To get funding as quickly as possible to the research community, we are using Urgent and Emergency competing revisions and administrative supplements to existing grant awards. This approach allows us to leverage resident expertise, getting additional funding to those researchers who are already working with other organisms, models, or tools so that they can quickly shift focus to the novel coronavirus. These Urgent and Emergency competitive revision Funding Opportunity Announcements (FOAs) allow NIH to fund applications quickly, often in under three months, sometimes much quicker than that, because evaluation for scientific and technical merit is done by an internal review panel convened by staff of the NIH awarding institute or center rather than by our traditional peer review process.
The Urgent and Emergency competing revision FOAs sound very similar. And they are, but there is an important distinction.
- The Emergency Competitive Revision FOA can only be used for funding available for applications based on a presidentially declared disaster under the Stafford Act, a public health emergency declared by the Secretary, HHS, or other local, regional or national disaster. This means that for COVID-19 funding, it can only be used by those NIH Institutes and Centers I listed above that received special emergency funding.
- The Urgent Competitive Revision FOA can be used to meet immediate needs to help address a specific public health crisis in a timely manner. This vehicle is used to help address a specific public health crisis that was unforeseen when the application or progress report was submitted.
When responding to these types of funding opportunities, it is important that you understand how they work.
- They require applications to be submitted in response to an Emergency or Urgent Notice of Special Interest (NOSI). We are maintaining a list of COVID-19 specific Notices of Special Interest on our Coronavirus Disease 2019 (COVID-19): Information for NIH Applicants and Recipients of NIH Funding website.
- You need to read the instructions in the NOSI and in the FOA it points to carefully. If the instructions in the NOSI differ from those in the FOA, follow those in the NOSI.
- There are specific review criteria specified in the FOA. Make sure you address those as well as any that might be mentioned in the NOSI. They are how NIH staff will evaluate your application for funding.
- The NOSI will instruct you to include the NOSI number in the Agency Routing Identifier field (Box 4b) of the SF424 (R&R) Form. This information is very important for NIH tracking of spending of emergency award funding. Applications without this information in Box 4b may not be considered for this type of funding.
- Often the due dates are rolling, meaning you should submit the application as soon as it is ready to get it considered for funding as quickly as possible.
NIH is issuing new COVID-19 related NOSIs frequently. Please check back for these and other COVID-19-related information on our Coronavirus Disease 2019 (COVID-19): Information for NIH Applicants and Recipients of NIH Funding website.
You can learn more about NOSI’s in this quick 5 minute video.

______________________________________________________
American Heart Association (AHA) COVID-19 Update
April 9, 2020
Dear AHA Research Community,
We know many of you are experiencing significant challenges conducting research at your institutions during the coronavirus pandemic. You no doubt have questions about steps the AHA is taking to support your research programs.
First and foremost is the safety of you and your research teams. We anticipate you are following the direction of your sponsoring institutions with regard to access to laboratories and research clinics. We are aware there are varying responses to the pandemic, ranging from all research activities being suspended indefinitely, to reduced operations and remote working until further notice. We understand the need for decisions to be made at the local level, with fluctuating degrees of interruptions on a daily and weekly basis. The AHA has adopted flexible grants management policies in recent years and will expand that flexibility and support as much as possible.
Disruptions to Ongoing Research Projects
- Inform AHA if your funded projects are placed on hold indefinitely. This could take the form of restrictions on human subject recruitment or visits with enrolled subjects, as well as inaccessibility to laboratories.
- AHA will support interim-year carryover requests due to suspension of research projects and allow rebudgeting.
Reporting
- Currently pending deadlines to submit scientific progress reports and patient recruitment/retention reports should proceed in a timely manner. The AHA is not extending these deadlines at this time.
- Contact awards@heart.org if submission of upcoming expenditure reports will be impacted by closure and/or reduced staffing of institutional accounting offices.
Award Extensions and Salary Expenditures During Work Stoppage
- No-cost-extensions (NCE) are available as always. Please submit a Change Request in Grants@Heart. Awards currently on NCE may qualify for additional extensions.
- AHA will also allow payment of salaries on grants and fellowships for up to 60 days without pre-approval during periods of work.
- stoppage. Should inability to conduct research exceed 60 days, please contact AHA.
Expenditures Related to Cancelled Travel
- Unrefunded charges (conference registration, airline ticket, etc.) incurred with the intent to attend a scientific conference that was subsequently cancelled can be charged to an AHA grant.
Deadlines for New Applications
- AHA’s next proposal deadlines for research funding will be in August.
- Any changes to upcoming deadlines will be announced by July 1.
______________________________________________________
Updates from the Research Administration Digest [RAD]
April 3, 2020
NSF Implementation OMB M-20-17 (COVID-19)
Refer to revisions released on April 1, 2020 regarding the implementation of OMB Memorandum M-20-17, “Administrative Relief for Recipients and Applicants of Federal Financial Assistance Directly Impacted by (COVID-19) due to Loss of Operations (March 19, 2020). NSF is continually updating guidance and online resources and is accepting proposals for nonmedical, non-clinical-care RAPID research on coronavirus. Visit https://www.nsf.gov/news/special_reports/coronavirus/.
NIH Late Application Policy (COVID-19)
NOT-OD-20-091 announced that grant applications submitted late (due dates between March 9, 2020, and May 1, 2020) will be accepted through May 1, 2020. This notice applies to all relevant funding opportunity announcements, including those that indicate no late applications accepted. A cover letter providing a justification is not required. NIH will be extending the expiration date of most FOAs expiring between now and May 1, 2020.
Research.gov (NSF Separately Submitted Proposals)
Effective March 30, 2020, "the research community can prepare and submit separately submitted collaborative proposals from multiple organizations in Research.gov," announced in a statement from Jean Feldman, NSF Head, Policy Office, Division of Institution and Award Support. Proposers can now prepare full, research proposals in Research.gov that are single submissions from one organization; single submission collaborative proposals with subawards and (a new feature) separately submitted collaborative proposals from multiple organizations. Read what's new for "Separately Submitted Collaborative Proposals." Visit Research.gov
SciENcv Biosketch Tool (NIH & NSF)
SciENcv (Science Experts Network Curriculum Vitae) is the most efficient way to create and maintain biosketches for NIH and NSF grant applications and annual reports. Use of an NSF-approved format for submission of these proposal sections is not required until implementation of the revised PAPPG on June 1, 2020. However, NSF is encouraging proposers to begin using the NSF-approved formats now. Read this notice to our community which includes resources and tools. Visit the SciENcv website and follow the link for the HMS step-by-step guide to Creating an NIH or NSF Biosketch in SciENcv (Located on the ORA website business process section). For additional questions, contact pernille_konow@hms.harvard.edu in the HMS Office of Research Administration.
CRI Fellowship Deadline Extended
In response to (COVID-19), the Cancer Research Institute extended the deadline to April 15, 2020, for the Irvington Postdoctoral Fellowship Program. Questions? Contact grants@cancerresearch.org. In an effort to be as flexible as possible, further requests for extension will be on a case-by-case basis
To subscribe to the RAD, visit the ORA website. Access the current and past issues here.
____________________________________________________________________________________________________________
NIH COVID-19 Info for NIH Applicants and Recipients
March 26, 2020
Responding to Frequent Questions on Flexibilities Related to NIH Funding and COVID-19
The public health emergency due to COVID-19 is causing difficulties in many aspects of our lives. My colleagues and I here at NIH are well aware of the challenges being felt in the research community as institutions are closing, people are being asked to practice social distancing, and resources and attention are justifiably focused on public health needs. We are listening to your concerns and are working quickly to develop answers to your many questions.
We recently updated our Coronavirus Disease 2019 (COVID-19): Information for NIH Applicants and Recipients website with a slew of additional FAQs, new funding opportunities, as well as the video message from me, below, where I address some of the most common questions.
Since yesterday’s recording of this video, in response to community concerns about their ability to submit applications in a timely manner, we have published a notice announcing that grant applications submitted late for due dates between March 9, 2020, and May 1, 2020, will be accepted through May 1, 2020. This notice applies to all relevant funding opportunity announcements, including those that indicate no late applications will be accepted. A cover letter providing a justification is not required. NIH will be extending the expiration date of most FOAs expiring between now and May 1. Be sure to read the notice carefully for details.
Things are moving quickly. Please continue to communicate with us. We are listening.
I encourage you to monitor our website frequently. To help you identify updated content, the page now includes a link to page update history so you can easily see what’s new
______________________________________________________
Research Measures in COVID-19 Relief & Stimulus Package
March 26, 2020
This communication highlights a few of the provisions in the third COVID-19 relief/response package that might be of particular interest to researchers. To read more, please download a section-by-section summary and the legislative text.
- NIST - $6 million to provide continuity of operations and to conduct research and measurement science to support the testing and treatment of coronavirus. Page 622 of the legislative text.
- NSF - $75 million to support research at molecular, cellular, physiological and ecological levels to better understand coronavirus genetics, modes of action, transmission, virulence, and population dynamics. It is expected that many of these funds will be available through the Rapid Response Research funding mechanism. Read Dear Colleague Letter here. Page 629.
- DOE Office of Science - $99.5 million for costs related to equipment, personnel, and operations to support research on the coronavirus, including support to provide access to user facilities. Page 654.
- EPA S&T - $1.5 million to support research efforts regarding coronavirus – and specifically, research on methods to reduce risks from environmental transmission via contaminated surfaces. Page 713.
- CDC - $4.3 billion to support federal, state, and local public health agencies to prevent, prepare for and respond to the coronavirus. Included in that amount is $500 million for global disease detection and emergency response, $500 million for public health data surveillance and analytics infrastructure modernization, and $300 million for the Infectious Diseases Rapid Response Reserve Fund, which supports immediate response activities during outbreaks. Page 728.
- NIH - The bill includes $945 million to support research and expand on prior research plans, including developing an improved understanding of the prevalence of COVID-19, its transmission and the natural history of infection, and novel approaches to diagnosing the disease and past infection, and developing countermeasures for the prevention and treatment of its various stages. Of this total, $103 million is allocated for NHLBI, $706 million NIAID, $60 million NIBIB, $36 million NCATS, $30 million OD, and $10 million for National Library of Medicine. When combined with the first supplemental, Congress has provided $1.78 billion for NIH research on COVID 19. Page 730.
- BARDA (Biomedical Advanced Research and Development Authority) - At least $3.5 billion to advance construction, manufacturing, and purchase of vaccines and therapeutics to the American people.
- NASA - $60 million to support NASA with resources for operational adjustments associated with mission delays caused by NASA center closures related to coronavirus. Page 628.
Also, the package includes a number of health-related authorizations, including the HRSA health professionals and nursing workforce development programs.
Kara A. HaasDirector of Federal Relations
Harvard Public Affairs and Communications
______________________________________________________
NIH COVID-19 Information for NIH Applicants and Recipients
March 20, 2020
Click link to view Dr. Mike Lauer, NIH Deputy Director for Extramural Research, provide an update for applicants and recipients of federal funding on flexibilities NIH has put in place to support them and their research through the disruptions caused by COVID-19.
The NIH's website on Coronavirus Disease 2019 (COVID-19): Information for NIH Applicants and Recipients has a list of available resources.
______________________________________________________
USAMRAA’s COVID-19 FAQs
March 18, 2020
This Notice addresses general questions associated with proposal submission and award management that may arise in relation to COVID-19. The United States Army Medical Research Acquisition Activity (USAMRAA) is providing this information as a service to our applicant and recipient communities to address immediate, high-level questions that have been posed to federal research assistance agencies. Please note that given the fact that COVID-19 and associated impacts continue to evolve, applicants and recipients are strongly encouraged to monitor the resources noted below for updates. USAMRAA is publishing information on flexibilities for organizations funded by USAMRAA to conduct research on COVID-19 along with Frequently Asked Questions (FAQs) on other administrative flexibilities whose operations have been adversely impacted in the emergency response related to COVID-19. These FAQs will be updated as more information becomes available.
1. What will be done for recipients whose awards support the continued research and services necessary to carry out the emergency response related to COVID-19 during the period formally declared by the Department of Health and Human Services through the 90 Day Public Health Emergency Declaration (Public Health Emergency Period)?
The Office of Management and Budget (OMB) has identified the following actions to relieve short-term administrative, financial management and audit requirements under the Uniform Guidance at 2 CFR Part 200 – “Uniform Administrative Requirements, Cost Principles, and Audit Requirements for Federal Awards”-without compromise to accountability requirements (M-20-11). USAMRAA will extend flexibilities to awards that support efforts related to COVID-19 on a case-by-case basis. Please contact the Grants Management Specialist or Grants Officer’s Representative listed in the award document for information concerning individual grants.
2. I have a question related to COVID-19's potential impact on my research project, project- related travel, or field work. Where are some of the places I can find helpful information?
Your employing organization is an ideal starting point. In many cases, colleges and universities have created websites offering information.
Beyond that, we encourage you to consult the following resources:
-
COVID-19 in general:
- Centers for Disease Control (CDC) including its guidance for Institutes of Higher Education
- World Health Organization (WHO)
- Local and state public health department
- Travel to/from and quarantine in foreign countries: See the State Department Travel Advisories website
- The Department of Defense’s Coronavirus Update
3. The DoD Research & Development (R&D) General Terms and Conditions provide the recipient the authority to extend the period of performance one time for up to 12 months beyond the original completion date down stated in the award document. Any additional project period extension beyond the initial extension of up to 12 months requires prior approval from the DoD awarding component.
Part 2, Article V., Section C (Financial and Program Management) of the R&D General Terms and Conditions addresses pre-award costs, carry forward of unobligated balances, and one-time no cost-extensions. Item 3 indicates that one-time no-cost extension provisions are reserved for the discretion of the DoD awarding component. See the agency-specific or award specific section of your award to see whether the awarding agency has already authorized a one-time no-cost extension without the need for prior approval. If it has not done so, once you have assessed how much additional time will be needed to complete performance, contact the POC on the award to request an extension. Grants officials have been advised to be flexible in considering requests related to this public health emergency.
4. I am an investigator on a USAMRAA-funded award that includes travel to a meeting/conference, but the meeting/conference has been canceled. Who do I contact regarding the impact to the USAMRAA award?
You should contact the cognizant USAMRAA grants official named in the award document to alert them to the situation. Also, copy the program official to ensure all appropriate federal staff are aware of the circumstances. In light of the public health threat, you may wish to consider alternate plans, such as providing or using options for virtual participation. Additionally, it may be possible to attend if the meeting/conference is rescheduled within a year. See the question above regarding one-time no-cost extensions.
For meetings that are specified directly in the application or award notice, or that have been required by the Congressionally Directed Medical Research Program (CDMRP) (e.g. Milestone Meetings, In-Progress Reviews, etc.), we will be open to rescheduling the meeting or setting up a virtual presentation.
5. A conference has been canceled, but I have nonrefundable travel, registration, and/or hotel costs. Can these be charged to a USAMRAA grant?
USAMRAA is currently working within DoD as well as with our federal partners on a number of proposal and award-related issues pertaining to COVID-19. We will communicate with the about these issues through updated FAQs as further information becomes available. In the meantime, please continue to follow your organization’s relevant travel policies and procedures.
6. I am involved with a USAMRAA award with a meeting/conference scheduled to take place in the coming weeks. Should I continue with plans for the meeting?
USAMRAA recommends reaching out to the conference organizer or host. They are best positioned to know the guidance at the event location. They may recommend having contingency plans if the event is ultimately cancelled or re-located, or might be planning to provide options for virtual participation. If you are the organizer, you should consider developing contingency plans.
We also suggest checking the State Department Travel Advisories website if the conference involves foreign travel.
7. My USAMRAA grant involves an exchange of researchers (including students) and/or other foreign travel. Should I continue with plans?
Travel logistics, accessibility, and health and safety considerations of the participants in an active research project should always be a foremost consideration. USAMRAA recommends consulting with your organization about its policies and procedures. You should consider approaching the planned researcher exchanges and/or other foreign travel with flexibility, and/or devising alternate plans including virtual collaboration as appropriate. As noted above, we understand that plans for active research projects may be disrupted, to the point of needing extensions on the original award durations. For foreign travel, you should consult the State Department Travel Advisories website.
8. I have plans to attend a large scientific gathering. Should I continue?
We recommend first consulting with your organization about its policies and practices. In addition, you may consider reaching out to the organizer or host of the scientific gathering. They are best positioned to know the guidance at the event location. They may have contingency plans if the event is ultimately canceled or re-located, or they might be planning to provide options for virtual participation. We also suggest checking the State Department Travel Advisories website if the gathering involves foreign travel.
9. (a) My organization is open, but I am quarantined for a period of time. There is an application submission deadline during my quarantine period and some essential materials are in my office. I am the PI. Can my organization receive an extension to the deadline? (b) My organization has asked staff to stay home for an undetermined period of time. How would I petition for an extension of an application deadline?
There are currently no plans to modify existing application deadline dates but please continue to monitor Grants.gov for any potential change(s) to an application deadline.
Please contact the CDMRP Help Desk at help@eBRAP.org or 301-682-5507 if you need further assistance.
This response may be updated at a later date depending on whether application review panel meeting dates are revised. Please check this page periodically for possible updates.
10. My position is funded through an USAMRAA award. The university will officially close until further notice as a result of the COVID-19 outbreak. Can the USAMRAA award be used to pay my employment costs?
The Department of Defense (DoD) will only allow recipients to charge salaries and benefits to currently active awards for work actually performed to meet the project activities, regardless of the location where those duties are performed (i.e., telework eligible). Some allowable activities may include -- e.g., data analysis, preparation of articles and papers based on the analysis of the research findings, monitoring subrecipients, care of research animals, direct charged administrative costs, etc. Additionally, such charges to the award should only be made when the work is performed within the recipient organization’s policies for allowable remote/telework and/or emergency operations.
Applicable indirect costs may be charged to all allowed costs.
11. The COVID-19 pandemic has impacted the conduct of my DoD-supported human subjects research protocol. What do I need to report to the USAMRDC Human Research Protection Office (HRPO)?
In addition to seeking local guidance from your human research protection program officials and reviewing Institutional Review Board (IRB), notify the HRPO in the following circumstances:
a) Amendments: Per the terms of your HRPO approval, substantive amendments require HRPO review and approval prior to implementation. The HRPO will not require pre-approval of amendments intended to minimize risk of COVID-19 exposure for research volunteers or study team members. You must follow your institution’s guidance or requirements for IRB review and approval for amendments and must provide documentation to the HRPO of all such actions in a prompt manner via email to the following address: usarmy.detrick.medcom-usamrmc.mbx.COVID-19@mail.mil.
b) Unanticipated Problems Involving Risks to Subjects or Others (UPIRTSOs): Per the terms of your HRPO approval and the Common Rule, UPIRTSOs must be promptly reported. This includes any UPIRTSOs related to the COVID-19 pandemic, such as inadvertent exposure of research subjects and/or study personnel, missed or delayed safety assessments due to the pandemic, inability to provide study product or conduct key research interventions, etc. UPIRTSOs related to the COVID-19 pandemic must be promptly reported to the reviewing IRB and the following HRPO mailbox: usarmy.detrick.medcom-usamrmc.mbx.COVID-19@mail.mil
c) Halting research: Promptly report any actions taken to halt the conduct of ongoing human subjects research (e.g. pausing new enrollment, canceling follow-up procedures with previously enrolled subjects, etc.) due to the COVID-19 pandemic to the reviewing IRB and the following HRPO mailbox: usarmy.detrick.medcom-usamrmc.mbx.COVID-19@mail.mil
d) Note: You must adhere to all other reporting and submission requirements specified in your HRPO approval memorandum.
If notification by electronic mail is not feasible, notifications can be made telephonically to 301-619-2165. If you have questions, you can contact 301-619-2165 or the HRPO mailbox atusarmy.detrick.medcom-usamrmc.mbx.COVID-19@mail.mil.
USAMRAA is currently working internally with DoD as well as with our federal partners on a number of proposal and award-related issues pertaining to COVID-19. USAMRAA will communicate with the community about this issue and will provide guidance as further information becomes available. Please check the USAMRAA website for updates.